RAPIDR: an analysis package for non-invasive prenatal testing of aneuploidy
- PMID: 24990604
- PMCID: PMC4184262
- DOI: 10.1093/bioinformatics/btu419
RAPIDR: an analysis package for non-invasive prenatal testing of aneuploidy
Abstract
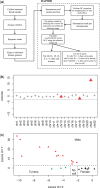
Non-invasive prenatal testing (NIPT) of fetal aneuploidy using cell-free fetal DNA is becoming part of routine clinical practice. RAPIDR (Reliable Accurate Prenatal non-Invasive Diagnosis R package) is an easy-to-use open-source R package that implements several published NIPT analysis methods. The input to RAPIDR is a set of sequence alignment files in the BAM format, and the outputs are calls for aneuploidy, including trisomies 13, 18, 21 and monosomy X as well as fetal sex. RAPIDR has been extensively tested with a large sample set as part of the RAPID project in the UK. The package contains quality control steps to make it robust for use in the clinical setting.
Availability and implementation: RAPIDR is implemented in R and can be freely downloaded via CRAN from here: http://cran.r-project.org/web/packages/RAPIDR/index.html.
Contact: kitty.lo@ucl.ac.uk
Supplementary information: Supplementary data are available at Bioinformatics online.
© The Author 2014. Published by Oxford University Press.
Figures
References
-
- Liang D, et al. Non-invasive prenatal testing of fetal whole chromosome aneuploidy by massively parallel sequencing. Prenat. Diagn. 2013;33:409–415. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous