Immune responses in macaques to a prototype recombinant adenovirus live oral human papillomavirus 16 vaccine
- PMID: 24990902
- PMCID: PMC4178560
- DOI: 10.1128/CVI.00197-14
Immune responses in macaques to a prototype recombinant adenovirus live oral human papillomavirus 16 vaccine
Abstract
Immunization with human papillomavirus (HPV) L1 virus-like particles (VLPs) prevents infection with HPV. However, the expense and logistical demands of current VLP vaccines will limit their widespread use in resource-limited settings, where most HPV-induced cervical cancer occurs. Live oral adenovirus vaccines have properties that are well-suited for use in such settings. We have described a live recombinant adenovirus vaccine prototype that produces abundant HPV16 L1 protein from the adenovirus major late transcriptional unit and directs the assembly of HPV16 VLPs in tissue culture. Recombinant-derived VLPs potently elicit neutralizing antibodies in mice. Here, we characterize the immune response to the recombinant after dual oral and intranasal immunization of pigtail macaques, in which the virus replicates as it would in immunized humans. The immunization of macaques induced vigorous humoral responses to adenovirus capsid and nonstructural proteins, although, surprisingly, not against HPV L1. In contrast, immunization elicited strong T-cell responses to HPV VLPs as well as adenovirus virions. T-cell responses arose immediately after the primary immunization and were boosted by a second immunization with recombinant virus. T-cell immunity contributes to protection against a wide variety of pathogens, including many viruses. The induction of a strong cellular response by the recombinant indicates that live adenovirus recombinants have potential as vaccines for those agents. These studies encourage and will inform the continued development of viable recombinant adenovirus vaccines.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

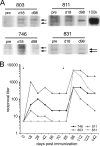
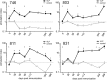
 ) are plotted for PBMCs drawn from each macaque at the indicated times. The arrows indicate immunizations; the third immunization was with purified HPV16 VLPs. The error bars represent the standard errors for the triplicate wells at each time point. The asterisks indicate the time points at which postimmunization and preimmunization responses differ statistically (P < 0.05). Interruptions in the plots indicate an extended period for which SI values were not determined. A horizontal line is drawn at an SI value of 1 for reference.
) are plotted for PBMCs drawn from each macaque at the indicated times. The arrows indicate immunizations; the third immunization was with purified HPV16 VLPs. The error bars represent the standard errors for the triplicate wells at each time point. The asterisks indicate the time points at which postimmunization and preimmunization responses differ statistically (P < 0.05). Interruptions in the plots indicate an extended period for which SI values were not determined. A horizontal line is drawn at an SI value of 1 for reference.
References
-
- Gaydos CA, Gray GC. 2008. Adenovirus vaccine, p 1103–1122 In Plotkin SA, Orenstein MD, Offit P. (ed), Vaccines, Saunders, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

