Prospective cohort study of the safety of an influenza A(H1N1) vaccine in pregnant Chinese women
- PMID: 24990911
- PMCID: PMC4178569
- DOI: 10.1128/CVI.00375-14
Prospective cohort study of the safety of an influenza A(H1N1) vaccine in pregnant Chinese women
Abstract
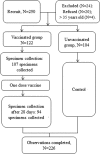
To monitor and evaluate the safety of the influenza A(H1N1) vaccine in pregnant women and its influence on the fetus and neonate, we performed a prospective study in which 122 pregnant Chinese women who received the influenza A(H1N1) vaccine and 104 pregnant women who did not receive any vaccine (serving as controls) were observed. The results indicated that the seroconversion rate in the vaccinated group was 90.4% (95% confidence interval [CI], 82.6% to 95.5%). The rate of adverse events following immunization in the pregnant women who received the influenza A(H1N1) vaccine was 3.3%. The spontaneous abortion rates in the vaccinated group and the unvaccinated group were 0.8% and 1.9%, respectively (exact probability test, P = 0.470), the prolonged-pregnancy rates were 8.2% and 4.8%, respectively (χ(2) = 1.041, P = 0.308), the low-birth-weight rates were 1.6% and 0.95%, respectively (exact probability test, P = 1.000), and the spontaneous-labor rates were 70.5% and 75%, respectively (χ(2) = 0.573, P = 0.449). All newborns who have an Apgar score of ≥7 are considered healthy; Apgar scores of ≥9 were observed in 38.5% and 57.7% of newborns in the vaccinated group and the unvaccinated group, respectively (χ(2) = 8.274, P = 0.004). From these results, we conclude that the influenza A(H1N1) vaccine is safe for pregnant women and has no observed adverse effects on fetal growth. (This study has been registered at ClinicalTrials.gov under registration no. NCT01842997.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Robertson LC, Allen SH, Konamme SP, Chestnut J, Wilson P. 2010. The successful use of extra-corporeal membrane oxygenation in the management of a pregnant woman with severe H1N1 2009 influenza complicated by pneumonitis and adult respiratory distress syndrome. Int. J. Obstet. Anesth. 19:443–447. 10.1016/j.ijoa.2010.04.010 - DOI - PMC - PubMed
-
- Bahloul M, Chaari A, Algia NB, Chtara K, Dammak H, Hamida CB, Kallel H, Chelly H, Bouaziz M. 2011. A literature review of respiratory failure following influenza A (H1N1) virus infection in pregnant and postpartum women. Trends Anaesth Crit. Care 1:252–256. 10.1016/j.tacc.2011.08.006 - DOI
-
- Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. 2011. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am. J. Obstet. Gynecol. 204:146.e141–e147. 10.1016/j.ajog.2010.08.050 - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical