Sleep disorders in Parkinsonian macaques: effects of L-dopa treatment and pedunculopontine nucleus lesion
- PMID: 24990932
- PMCID: PMC4078088
- DOI: 10.1523/JNEUROSCI.0181-14.2014
Sleep disorders in Parkinsonian macaques: effects of L-dopa treatment and pedunculopontine nucleus lesion
Abstract
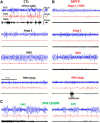
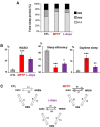
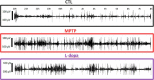
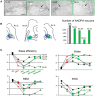
Patients with Parkinson's disease (PD) display significant sleep disturbances and daytime sleepiness. Dopaminergic treatment dramatically improves PD motor symptoms, but its action on sleep remains controversial, suggesting a causal role of nondopaminergic lesions in these symptoms. Because the pedunculopontine nucleus (PPN) regulates sleep and arousal, and in view of the loss of its cholinergic neurons in PD, the PPN could be involved in these sleep disorders. The aims of this study were as follows: (1) to characterize sleep disorders in a monkey model of PD; (2) to investigate whether l-dopa treatment alleviates sleep disorders; and (3) to determine whether a cholinergic PPN lesion would add specific sleep alterations. To this end, long-term continuous electroencephalographic monitoring of vigilance states was performed in macaques, using an implanted miniaturized telemetry device. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment induced sleep disorders that comprised sleep episodes during daytime and sleep fragmentation and a reduction of sleep efficiency at nighttime. It also induced a reduction in time spent in rapid eye movement (REM) sleep and slow-wave sleep and an increase in muscle tone during REM and non-REM sleep episodes and in the number of awakenings and movements. l-Dopa treatment resulted in a partial but significant improvement of almost all sleep parameters. PPN lesion induced a transient decrease in REM sleep and in slow-wave sleep followed by a slight improvement of sleep quality. Our data demonstrate the efficacy of l-dopa treatment in improving sleep disorders in parkinsonian monkeys, and that adding a cholinergic PPN lesion improves sleep quality after transient sleep impairment.
Keywords: Parkinson's disease; cholinergic neurons; l-dopa; macaques; pedunculopontine nucleus; sleep disorders.
Copyright © 2014 the authors 0270-6474/14/349124-10$15.00/0.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
