Direct evidence of an elongation factor-Tu/Ts·GTP·Aminoacyl-tRNA quaternary complex
- PMID: 24990941
- PMCID: PMC4156062
- DOI: 10.1074/jbc.M114.583385
Direct evidence of an elongation factor-Tu/Ts·GTP·Aminoacyl-tRNA quaternary complex
Abstract
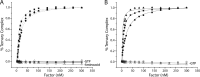
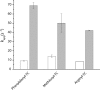
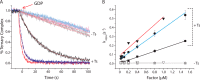
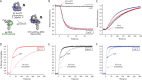
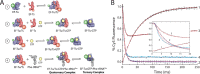
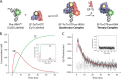
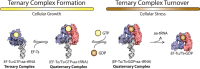
During protein synthesis, elongation factor-Tu (EF-Tu) bound to GTP chaperones the entry of aminoacyl-tRNA (aa-tRNA) into actively translating ribosomes. In so doing, EF-Tu increases the rate and fidelity of the translation mechanism. Recent evidence suggests that EF-Ts, the guanosine nucleotide exchange factor for EF-Tu, directly accelerates both the formation and dissociation of the EF-Tu-GTP-Phe-tRNA(Phe) ternary complex (Burnett, B. J., Altman, R. B., Ferrao, R., Alejo, J. L., Kaur, N., Kanji, J., and Blanchard, S. C. (2013) J. Biol. Chem. 288, 13917-13928). A central feature of this model is the existence of a quaternary complex of EF-Tu/Ts·GTP·aa-tRNA(aa). Here, through comparative investigations of phenylalanyl, methionyl, and arginyl ternary complexes, and the development of a strategy to monitor their formation and decay using fluorescence resonance energy transfer, we reveal the generality of this newly described EF-Ts function and the first direct evidence of the transient quaternary complex species. These findings suggest that EF-Ts may regulate ternary complex abundance in the cell through mechanisms that are distinct from its guanosine nucleotide exchange factor functions.
Keywords: Elongation Factor Ts; Elongation Factor Tu; G Protein; Guanine Nucleotide Exchange Factor (GEF); Guanosine Nucleotide Exchange Factor; Protein Synthesis; Ternary Complex; Translation; Translation Elongation Factor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Vetter I. R., Wittinghofer A. (2001) The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 - PubMed
-
- Leipe D. D., Wolf Y. I., Koonin E. V., Aravind L. (2002) Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317, 41–72 - PubMed
-
- Thomposon R. C., Dix D. B. (1982) Accuracy of protein biosynthesis. A kinetic study of the reaction of poly(U)-programmed ribosomes with a leucyl-tRNA2-elongation factor Tu-GTP complex. J. Biol. Chem. 257, 6677–6682 - PubMed
-
- Rodnina M. V., Pape T., Fricke R., Wintermeyer W. (1995) Elongation factor Tu, a GTPase triggered by codon recognition on the ribosome: mechanism and GTP consumption. Biochem. Cell Biol. 73, 1221–1227 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

