Akt-dependent activation of mTORC1 complex involves phosphorylation of mTOR (mammalian target of rapamycin) by IκB kinase α (IKKα)
- PMID: 24990947
- PMCID: PMC4155685
- DOI: 10.1074/jbc.M114.554881
Akt-dependent activation of mTORC1 complex involves phosphorylation of mTOR (mammalian target of rapamycin) by IκB kinase α (IKKα)
Abstract
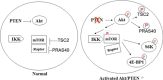
The serine/threonine protein kinase Akt promotes cell survival, growth, and proliferation through phosphorylation of different downstream substrates. A key effector of Akt is the mammalian target of rapamycin (mTOR). Akt is known to stimulate mTORC1 activity through phosphorylation of tuberous sclerosis complex 2 (TSC2) and PRAS40, both negative regulators of mTOR activity. We previously reported that IκB kinase α (IKKα), a component of the kinase complex that leads to NF-κB activation, plays an important role in promoting mTORC1 activity downstream of activated Akt. Here, we demonstrate IKKα-dependent regulation of mTORC1 using multiple PTEN null cancer cell lines and an animal model with deletion of IKKα. Importantly, IKKα is shown to phosphorylate mTOR at serine 1415 in a manner dependent on Akt to promote mTORC1 activity. These results demonstrate that IKKα is an effector of Akt in promoting mTORC1 activity.
Keywords: Akt; Cell Proliferation; IKK; Mammalian Target of Rapamycin (mTOR); Phosphatase and Tensin Homolog (PTEN); Phosphorylation; Raptor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

