Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR)
- PMID: 24990952
- PMCID: PMC4132766
- DOI: 10.1074/jbc.R114.566091
Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR)
Abstract
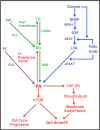
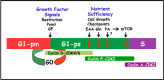
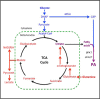
Phosphatidic acid (PA) is a critical metabolite at the heart of membrane phospholipid biosynthesis. However, PA also serves as a critical lipid second messenger that regulates several proteins implicated in the control of cell cycle progression and cell growth. Three major metabolic pathways generate PA: phospholipase D (PLD), diacylglycerol kinase (DGK), and lysophosphatidic acid acyltransferase (LPAAT). The LPAAT pathway is integral to de novo membrane phospholipid biosynthesis, whereas the PLD and DGK pathways are activated in response to growth factors and stress. The PLD pathway is also responsive to nutrients. A key target for the lipid second messenger function of PA is mTOR, the mammalian/mechanistic target of rapamycin, which integrates both nutrient and growth factor signals to control cell growth and proliferation. Although PLD has been widely implicated in the generation of PA needed for mTOR activation, it is becoming clear that PA generated via the LPAAT and DGK pathways is also involved in the regulation of mTOR. In this minireview, we highlight the coordinated maintenance of intracellular PA levels that regulate mTOR signals stimulated by growth factors and nutrients, including amino acids, lipids, glucose, and Gln. Emerging evidence indicates compensatory increases in one source of PA when another source is compromised, highlighting the importance of being able to adapt to stressful conditions that interfere with PA production. The regulation of PA levels has important implications for cancer cells that depend on PA and mTOR activity for survival.
Keywords: Diacylglycerol Kinase; Lipid Metabolism; Lysophosphatidic Acid Acyltransferase; Mammalian Target of Rapamycin (mTOR); Phosphatidic Acid; Phospholipase D; glycolysis.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

