A fly trap mechanism provides sequence-specific RNA recognition by CPEB proteins
- PMID: 24990967
- PMCID: PMC4083092
- DOI: 10.1101/gad.241133.114
A fly trap mechanism provides sequence-specific RNA recognition by CPEB proteins
Abstract
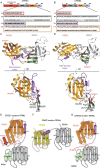
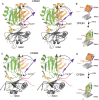
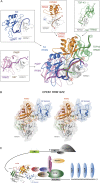
Cytoplasmic changes in polyA tail length is a key mechanism of translational control and is implicated in germline development, synaptic plasticity, cellular proliferation, senescence, and cancer progression. The presence of a U-rich cytoplasmic polyadenylation element (CPE) in the 3' untranslated regions (UTRs) of the responding mRNAs gives them the selectivity to be regulated by the CPE-binding (CPEB) family of proteins, which recognizes RNA via the tandem RNA recognition motifs (RRMs). Here we report the solution structures of the tandem RRMs of two human paralogs (CPEB1 and CPEB4) in their free and RNA-bound states. The structures reveal an unprecedented arrangement of RRMs in the free state that undergo an original closure motion upon RNA binding that ensures high fidelity. Structural and functional characterization of the ZZ domain (zinc-binding domain) of CPEB1 suggests a role in both protein-protein and protein-RNA interactions. Together with functional studies, the structures reveal how RNA binding by CPEB proteins leads to an optimal positioning of the N-terminal and ZZ domains at the 3' UTR, which favors the nucleation of the functional ribonucleoprotein complexes for translation regulation.
Keywords: CPEB1; CPEB4; binuclear zinc-binding domain; cytoplasmic polyadenylation; protein–RNA interactions; translational regulation.
© 2014 Afroz et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Alexandrov IM, Ivshina M, Jung DY, Friedline R, Ko HJ, Xu M, O’Sullivan-Murphy B, Bortell R, Huang YT, Urano F, et al. 2012. Cytoplasmic polyadenylation element binding protein deficiency stimulates PTEN and Stat3 mRNA translation and induces hepatic insulin resistance. PLoS Genet 8: e1002457. - PMC - PubMed
-
- Allain FH, Gilbert DE, Bouvet P, Feigon J 2000. Solution structure of the two N-terminal RNA-binding domains of nucleolin and NMR study of the interaction with its RNA target. J Mol Biol 303: 227–241 - PubMed
-
- Barraud P, Allain FH 2013. Solution structure of the two RNA recognition motifs of hnRNP A1 using segmental isotope labeling: how the relative orientation between RRMs influences the nucleic acid binding topology. J Biol NMR 55: 119–138 - PubMed
-
- Bava FA, Eliscovich C, Ferreira PG, Minana B, Ben-Dov C, Guigo R, Valcarcel J, Mendez R 2013. CPEB1 coordinates alternative 3′-UTR formation with translational regulation. Nature 495: 121–125 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
