Unexpected structural features of the hepatitis C virus envelope protein 2 ectodomain
- PMID: 24991010
- PMCID: PMC4178838
- DOI: 10.1128/JVI.00874-14
Unexpected structural features of the hepatitis C virus envelope protein 2 ectodomain
Abstract
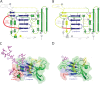
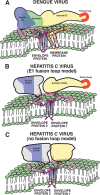
Hepatitis C virus (HCV), a member of the family Flaviviridae, is a leading cause of chronic liver disease and cancer. Recent advances in HCV therapeutics have resulted in improved cure rates, but an HCV vaccine is not available and is urgently needed to control the global pandemic. Vaccine development has been hampered by the lack of high-resolution structural information for the two HCV envelope glycoproteins, E1 and E2. Recently, Kong and coworkers (Science 342:1090-1094, 2013, doi:10.1126/science.1243876) and Khan and coworkers (Nature 509[7500]:381-384, 2014, doi:10.1038/nature13117) independently determined the structure of the HCV E2 ectodomain core with some unexpected and informative results. The HCV E2 ectodomain core features a globular architecture with antiparallel β-sheets forming a central β sandwich. The residues comprising the epitopes of several neutralizing and nonneutralizing human monoclonal antibodies were also determined, which is an essential step toward obtaining a fine map of the human humoral response to HCV. Also clarified were the regions of E2 that directly bind CD81, an important HCV cellular receptor. While it has been widely assumed that HCV E2 is a class II viral fusion protein (VFP), the newly determined structure suggests that the HCV E2 ectodomain shares structural and functional similarities only with domain III of class II VFPs. The new structural determinations suggest that the HCV glycoproteins use a different mechanism than that used by class II fusion proteins for cell fusion.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Identification of conserved residues in hepatitis C virus envelope glycoprotein E2 that modulate virus dependence on CD81 and SRB1 entry factors.J Virol. 2014 Sep;88(18):10584-97. doi: 10.1128/JVI.01402-14. Epub 2014 Jul 2. J Virol. 2014. PMID: 24990994 Free PMC article.
-
Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2.Nature. 2014 May 15;509(7500):381-4. doi: 10.1038/nature13117. Epub 2014 Feb 19. Nature. 2014. PMID: 24553139 Free PMC article.
-
A Recombinant Hepatitis C Virus Genotype 1a E1/E2 Envelope Glycoprotein Vaccine Elicits Antibodies That Differentially Neutralize Closely Related 2a Strains through Interactions of the N-Terminal Hypervariable Region 1 of E2 with Scavenger Receptor B1.J Virol. 2019 Oct 29;93(22):e00810-19. doi: 10.1128/JVI.00810-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462563 Free PMC article.
-
Structure and Function of the Hepatitis C Virus Envelope Glycoproteins E1 and E2: Antiviral and Vaccine Targets.ACS Infect Dis. 2016 Nov 11;2(11):749-762. doi: 10.1021/acsinfecdis.6b00110. Epub 2016 Aug 16. ACS Infect Dis. 2016. PMID: 27933781 Review.
-
Computational Modeling of Hepatitis C Virus Envelope Glycoprotein Structure and Recognition.Front Immunol. 2018 May 28;9:1117. doi: 10.3389/fimmu.2018.01117. eCollection 2018. Front Immunol. 2018. PMID: 29892287 Free PMC article. Review.
Cited by
-
HCV-E2 inhibits hepatocellular carcinoma metastasis by stimulating mast cells to secrete exosomal shuttle microRNAs.Oncol Lett. 2017 Aug;14(2):2141-2146. doi: 10.3892/ol.2017.6433. Epub 2017 Jun 20. Oncol Lett. 2017. Retraction in: Oncol Lett. 2024 May 08;28(1):301. doi: 10.3892/ol.2024.14434. PMID: 28781655 Free PMC article. Retracted.
-
Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis.PLoS Pathog. 2017 Dec 18;13(12):e1006735. doi: 10.1371/journal.ppat.1006735. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29253863 Free PMC article.
-
Antibody Repertoire Analysis of Hepatitis C Virus Infections Identifies Immune Signatures Associated With Spontaneous Clearance.Front Immunol. 2018 Dec 21;9:3004. doi: 10.3389/fimmu.2018.03004. eCollection 2018. Front Immunol. 2018. PMID: 30622532 Free PMC article.
-
Functional Analysis of Hepatitis C Virus (HCV) Envelope Protein E1 Using a trans-Complementation System Reveals a Dual Role of a Putative Fusion Peptide of E1 in both HCV Entry and Morphogenesis.J Virol. 2017 Mar 13;91(7):e02468-16. doi: 10.1128/JVI.02468-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28100619 Free PMC article.
-
Computational Prediction of the Heterodimeric and Higher-Order Structure of gpE1/gpE2 Envelope Glycoproteins Encoded by Hepatitis C Virus.J Virol. 2017 Mar 29;91(8):e02309-16. doi: 10.1128/JVI.02309-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148799 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R41AI068230/AI/NIAID NIH HHS/United States
- R41 AI068230/AI/NIAID NIH HHS/United States
- UC1AI067188/AI/NIAID NIH HHS/United States
- R56 AI64617/AI/NIAID NIH HHS/United States
- R21 DK070551/DK/NIDDK NIH HHS/United States
- R21AI097809/AI/NIAID NIH HHS/United States
- UC1 AI067188/AI/NIAID NIH HHS/United States
- R21 AI092073/AI/NIAID NIH HHS/United States
- DK070551/DK/NIDDK NIH HHS/United States
- R21 AI097809/AI/NIAID NIH HHS/United States
- R56 AI064617/AI/NIAID NIH HHS/United States
- R21AI092073/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

