Homeostatic cytokines induce CD4 downregulation in African green monkeys independently of antigen exposure to generate simian immunodeficiency virus-resistant CD8αα T cells
- PMID: 24991011
- PMCID: PMC4178902
- DOI: 10.1128/JVI.01331-14
Homeostatic cytokines induce CD4 downregulation in African green monkeys independently of antigen exposure to generate simian immunodeficiency virus-resistant CD8αα T cells
Abstract
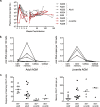
African green monkeys (AGMs; genus Chlorocebus) are a natural host of simian immunodeficiency virus (SIVAGM). As they do not develop simian AIDS, there is great interest in understanding how this species has evolved to avoid immunodeficiency. Adult African green monkeys naturally have low numbers of CD4 T cells and a large population of major histocompatibility complex class II-restricted CD8α(dim) T cells that are generated through CD4 downregulation in CD4(+) T cells. Mechanisms that drive this process of CD4 downregulation are unknown. Here, we show that juvenile AGMs accelerate CD4-to-CD8αα conversion upon SIV infection and avoid progression to AIDS. The CD4 downregulation induced by SIV infection is not limited to SIV-specific T cells, and vaccination of an adult AGM who had a negligible number of CD4 T cells demonstrated that CD4 downregulation can occur without antigenic exposure. Finally, we show that the T cell homeostatic cytokines interleukin-2 (IL-2), IL-7, and IL-15 can induce CD4 downregulation in vitro. These data identify a mechanism that allows AGMs to generate a large, diverse population of T cells that perform CD4 T cell functions but are resistant to SIV infection. A better understanding of this mechanism may allow the development of treatments to induce protective CD4 downregulation in humans.
Importance: Many African primate species are naturally infected with SIV. African green monkeys, one natural host species, avoid simian AIDS by creating a population of T cells that lack CD4, the human immunodeficiency virus/SIV receptor; therefore, they are resistant to infection. However, these T cells maintain properties of CD4(+) T cells even after receptor downregulation and preserve immune function. Here, we show that juvenile AGMs, who have not undergone extensive CD4 downregulation, accelerate this process upon SIV infection. Furthermore, we show that in vivo, CD4 downregulation does not occur exclusively in antigen-experienced T cells. Finally, we show that the cytokines IL-2, IL-7, and IL-15, which induce homeostatic T cell proliferation, lead to CD4 downregulation in vitro; therefore, they can provide signals that lead to antigen-independent CD4 downregulation. These results suggest that if a similar process of CD4 downregulation could be induced in humans, it could provide a cure for AIDS.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. 2005. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J. Virol. 79:5153–5162. 10.1128/JVI.79.8.5153-5162.2005 - DOI - PMC - PubMed
-
- Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955–967 - PMC - PubMed
-
- Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15:879–885. 10.1038/nm.1970 - DOI - PMC - PubMed
-
- Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035–3046. 10.4049/jimmunol.179.5.3035 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

