β-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice
- PMID: 24991237
- PMCID: PMC4078260
- DOI: 10.1186/alzrt262
β-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice
Abstract
Introduction: Vascular dementia is the second most common cause of dementia after Alzheimer's disease (AD). In addition, it is estimated that almost half of all AD patients have significant cerebrovascular disease comorbid with their AD pathology. We hypothesized that cerebrovascular disease significantly impacts AD pathological progression.
Methods: We used a dietary model of cerebrovascular disease that relies on the induction of hyperhomocysteinemia (HHcy). HHcy is a significant clinical risk factor for stroke, cardiovascular disease and type 2 diabetes. In the present study, we induced HHcy in APP/PS1 transgenic mice.
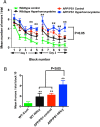
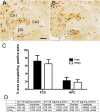
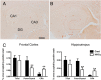
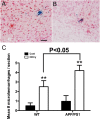
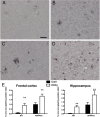
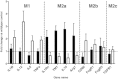
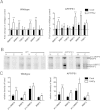
Results: While total β-amyloid (Aβ) load is unchanged across groups, Congophilic amyloid deposition was decreased in the parenchyma and significantly increased in the vasculature as cerebral amyloid angiopathy (CAA; vascular amyloid deposition) in HHcy APP/PS1 mice. We also found that HHcy induced more microhemorrhages in the APP/PS1 mice than in the wild-type mice and that it switched the neuroinflammatory phenotype from an M2a biased state to an M1 biased state. Associated with these changes was an induction of the matrix metalloproteinase protein 2 (MMP2) and MMP9 systems. Interestingly, after 6 months of HHcy, the APP/PS1 mice were cognitively worse than wild-type HHcy mice or APP/PS1 mice, indicative of an additive effect of the cerebrovascular pathology and amyloid deposition.
Conclusions: These data show that cerebrovascular disease can significantly impact Aβ distribution in the brain, favoring vascular deposition. We predict that the presence of cerebrovascular disease with AD will have a significant impact on AD progression and the efficacy of therapeutics.
Figures
References
-
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. on behalf of the American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. - PMC - PubMed
-
- Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431–1438. - PubMed
-
- Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–2908. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

