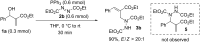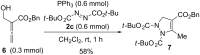Regioselective SN2' Mitsunobu reaction of Morita-Baylis-Hillman alcohols: A facile and stereoselective synthesis of α-alkylidene-β-hydrazino acid derivatives
- PMID: 24991249
- PMCID: PMC4077529
- DOI: 10.3762/bjoc.10.98
Regioselective SN2' Mitsunobu reaction of Morita-Baylis-Hillman alcohols: A facile and stereoselective synthesis of α-alkylidene-β-hydrazino acid derivatives
Abstract
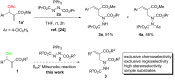
A highly regioselective SN2' Mitsunobu reaction between Morita-Baylis-Hillman (MBH) alcohols, azodicarboxylates, and triphenylphosphine is developed, which provides an easy access to α-alkylidene-β-hydrazino acid derivatives in high yields and good stereoselectivity. This reaction represents the first direct transformation of MBH alcohols into hydrazines.
Keywords: Mitsunobu reaction; Morita–Baylis–Hillman; SN2' reaction; azodicarboxylate; hydrazine.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases