The Ugi four-component reaction as a concise modular synthetic tool for photo-induced electron transfer donor-anthraquinone dyads
- PMID: 24991251
- PMCID: PMC4077531
- DOI: 10.3762/bjoc.10.100
The Ugi four-component reaction as a concise modular synthetic tool for photo-induced electron transfer donor-anthraquinone dyads
Abstract
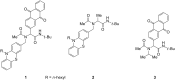
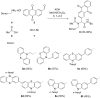
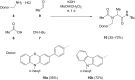
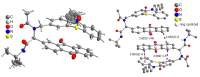
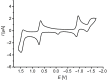
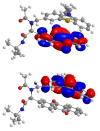
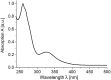
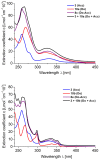
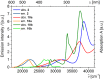
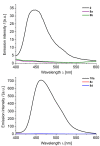
Phenothiazinyl and carbazolyl-donor moieties can be covalently coupled to an anthraquinone acceptor unit through an Ugi four-component reaction in a rapid, highly convergent fashion and with moderate to good yields. These novel donor-acceptor dyads are electronically decoupled in the electronic ground state according to UV-vis spectroscopy and cyclic voltammetry. However, in the excited state the inherent donor luminescence is efficiently quenched. Previously performed femtosecond spectroscopic measurements account for a rapid exergonic depopulation of the excited singlet states into a charge-separated state. Calculations of the Gibbs energy of photo-induced electron transfer from readily available UV-vis spectroscopic and cyclovoltammetric data applying the Weller approximation enables a quick evaluation of these novel donor-acceptor dyads. In addition, the X-ray structure of a phenothiazinyl-anthraquinone dyad supports short donor-acceptor distances by an intramolecular π-stacking conformation, an important assumption also implied in the calculations of the Gibbs energies according to the Weller approximation.
Keywords: absorption spectroscopy; chromophores; cyclic voltammetry; fluorescence; multicomponent reactions; photo-induced electron transfer.
Figures
Similar articles
-
Influence of π-conjugation structural changes on intramolecular charge transfer and photoinduced electron transfer in donor-π-acceptor dyads.Phys Chem Chem Phys. 2016 Dec 21;19(1):426-435. doi: 10.1039/c6cp06566j. Phys Chem Chem Phys. 2016. PMID: 27905585
-
The influence of π-conjugation on competitive pathways: charge transfer or electron transfer in new D-π-A and D-π-Si-π-A dyads.Phys Chem Chem Phys. 2016 Aug 17;18(33):22921-8. doi: 10.1039/c6cp03259a. Phys Chem Chem Phys. 2016. PMID: 27485173
-
Design, synthesis, and photophysical studies of a porphyrin-fullerene dyad with parachute topology; charge recombination in the marcus inverted region.J Am Chem Soc. 2004 Jun 16;126(23):7257-70. doi: 10.1021/ja038676s. J Am Chem Soc. 2004. PMID: 15186163
-
Quadrupolar Cyclopenta[hi]aceanthrylene-Based Electron Donor-Acceptor-Donor Conjugates: Charge Transfer versus Charge Separation.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14644-14652. doi: 10.1002/anie.201906206. Epub 2019 Sep 5. Angew Chem Int Ed Engl. 2019. PMID: 31381224 Review.
-
Long-lived charge separation and applications in artificial photosynthesis.Acc Chem Res. 2014 May 20;47(5):1455-64. doi: 10.1021/ar400200u. Epub 2014 May 5. Acc Chem Res. 2014. PMID: 24793793 Review.
Cited by
-
Review on the Ugi Multicomponent Reaction Mechanism and the Use of Fluorescent Derivatives as Functional Chromophores.ACS Omega. 2020 Jan 7;5(2):972-979. doi: 10.1021/acsomega.9b03684. eCollection 2020 Jan 21. ACS Omega. 2020. PMID: 31984252 Free PMC article. Review.
-
Modular Design and Scaffold-Synthesis of Multi-Functional Fluorophores for Targeted Cellular Imaging and Pyroptosis.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202415627. doi: 10.1002/anie.202415627. Epub 2024 Nov 18. Angew Chem Int Ed Engl. 2025. PMID: 39555698 Free PMC article.
-
Unimolecular Exciplexes by Ugi Four-Component Reaction.Front Chem. 2019 Nov 1;7:717. doi: 10.3389/fchem.2019.00717. eCollection 2019. Front Chem. 2019. PMID: 31737597 Free PMC article.
-
Thiophene-forming one-pot synthesis of three thienyl-bridged oligophenothiazines and their electronic properties.Beilstein J Org Chem. 2016 Sep 20;12:2055-2064. doi: 10.3762/bjoc.12.194. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27829911 Free PMC article.
-
Multicomponent synthesis of chromophores - The one-pot approach to functional π-systems.Front Chem. 2023 Mar 17;11:1124209. doi: 10.3389/fchem.2023.1124209. eCollection 2023. Front Chem. 2023. PMID: 37007054 Free PMC article. Review.
References
-
- Müller T J J, Bunz U H F, editors. Functional Organic Materials. Syntheses, Strategies, and Applications. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2007.
-
- Müllen K, Wegner G, editors. Electronic Materials: The Oligomer Approach. Weinheim, Germany: Wiley-VCH; 1998.
-
- Müllen K, Scherf U, editors. Organic Light-Emitting Diodes – Synthesis, Properties, and Applications. Weinheim, Germany: Wiley-VCH; 2006.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
