Direct C-H trifluoromethylation of di- and trisubstituted alkenes by photoredox catalysis
- PMID: 24991259
- PMCID: PMC4077392
- DOI: 10.3762/bjoc.10.108
Direct C-H trifluoromethylation of di- and trisubstituted alkenes by photoredox catalysis
Abstract
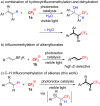
Background: Trifluoromethylated alkene scaffolds are known as useful structural motifs in pharmaceuticals and agrochemicals as well as functional organic materials. But reported synthetic methods usually require multiple synthetic steps and/or exhibit limitation with respect to access to tri- and tetrasubstituted CF3-alkenes. Thus development of new methodologies for facile construction of Calkenyl-CF3 bonds is highly demanded.
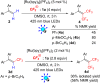
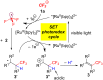
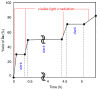
Results: The photoredox reaction of alkenes with 5-(trifluoromethyl)dibenzo[b,d]thiophenium tetrafluoroborate, Umemoto's reagent, as a CF3 source in the presence of [Ru(bpy)3](2+) catalyst (bpy = 2,2'-bipyridine) under visible light irradiation without any additive afforded CF3-substituted alkenes via direct Calkenyl-H trifluoromethylation. 1,1-Di- and trisubstituted alkenes were applicable to this photocatalytic system, providing the corresponding multisubstituted CF3-alkenes. In addition, use of an excess amount of the CF3 source induced double C-H trifluoromethylation to afford geminal bis(trifluoromethyl)alkenes.
Conclusion: A range of multisubstituted CF3-alkenes are easily accessible by photoredox-catalyzed direct C-H trifluoromethylation of alkenes under mild reaction conditions. In particular, trifluoromethylation of triphenylethene derivatives, from which synthetically valuable tetrasubstituted CF3-alkenes are obtained, have never been reported so far. Remarkably, the present facile and straightforward protocol is extended to double trifluoromethylation of alkenes.
Keywords: electrophilic trifluoromethylating reagent; multi-substituted alkene; photoredox catalysis; radical reaction; trifluoromethylation.
Figures


Similar articles
-
Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon-Carbon Multiple Bonds.Acc Chem Res. 2016 Sep 20;49(9):1937-45. doi: 10.1021/acs.accounts.6b00268. Epub 2016 Aug 26. Acc Chem Res. 2016. PMID: 27564676
-
Photoredox-Catalyzed Stereoselective Conversion of Alkynes into Tetrasubstituted Trifluoromethylated Alkenes.Angew Chem Int Ed Engl. 2015 Oct 26;54(44):12923-7. doi: 10.1002/anie.201505550. Epub 2015 Sep 11. Angew Chem Int Ed Engl. 2015. PMID: 26360134
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Direct C-H Trifluoromethylation of Glycals by Photoredox Catalysis.Org Lett. 2015 Nov 20;17(22):5698-701. doi: 10.1021/acs.orglett.5b03016. Epub 2015 Nov 12. Org Lett. 2015. PMID: 26562610
-
Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents.Org Biomol Chem. 2014 Sep 14;12(34):6580-9. doi: 10.1039/c4ob00671b. Org Biomol Chem. 2014. PMID: 25011917 Review.
Cited by
-
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups.Beilstein J Org Chem. 2019 Sep 23;15:2213-2270. doi: 10.3762/bjoc.15.218. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31598178 Free PMC article. Review.
-
Cyanine-based near infra-red organic photoredox catalysis.Chem Sci. 2021 Apr 13;12(20):6964-6968. doi: 10.1039/d1sc00998b. Chem Sci. 2021. PMID: 34123323 Free PMC article.
-
Ruthenium-Catalyzed Site-Selective Trifluoromethylations and (Per)Fluoroalkylations of Anilines and Indoles.Chemistry. 2020 May 26;26(30):6784-6788. doi: 10.1002/chem.202001439. Epub 2020 May 12. Chemistry. 2020. PMID: 32216068 Free PMC article.
-
Dawn of photoredox catalysis.Proc Jpn Acad Ser B Phys Biol Sci. 2025;101(5):274-301. doi: 10.2183/pjab.101.019. Proc Jpn Acad Ser B Phys Biol Sci. 2025. PMID: 40350302 Free PMC article. Review.
-
Recent advances in direct trifluoromethylation of olefinic C-H bonds.RSC Adv. 2019 Sep 3;9(47):27625-27639. doi: 10.1039/c9ra04170b. eCollection 2019 Aug 29. RSC Adv. 2019. PMID: 35529210 Free PMC article. Review.
References
-
- Hiyama T. In: Organofluorine Compounds: Chemistry and Applications. Yamamoto H, editor. Berlin: Springer; 2000. - DOI
-
- Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Chichester: Wiley-Blackwell; 2009.
-
- Ma J-A, Cahard D. J Fluorine Chem. 2007;128:975–996. doi: 10.1016/j.jfluchem.2007.04.026. - DOI
-
- Shibata N, Mizuta S, Toru T. J Synth Org Chem, Jpn. 2008;66:215–228.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
