Stereocontrolled synthesis of 5-azaspiro[2.3]hexane derivatives as conformationally "frozen" analogues of L-glutamic acid
- PMID: 24991261
- PMCID: PMC4077356
- DOI: 10.3762/bjoc.10.110
Stereocontrolled synthesis of 5-azaspiro[2.3]hexane derivatives as conformationally "frozen" analogues of L-glutamic acid
Abstract
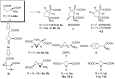
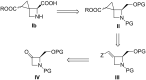
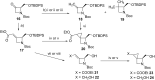
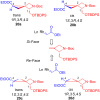
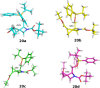
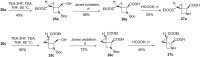
Several strategies aimed to "freeze" natural amino acids into more constrained analogues have been developed with the aim of enhancing in vitro potency/selectivity and, more in general, drugability properties. The case of L-glutamic acid (L-Glu, 1) is of particular importance since it is the primary excitatory neurotransmitter in the mammalian central nervous system (CNS) and plays a critical role in a wide range of disorders like schizophrenia, depression, neurodegenerative diseases such as Parkinson's and Alzheimer's and in the identification of new potent and selective ligands of ionotropic and metabotropic glutamate receptors (GluRs). To this aim, bicycle compound Ib was designed and synthesised from D-serine as novel [2.3]-spiro analogue of L-Glu. This frozen amino acid derivative was designed to further limit the rotation around the C3-C4 bond present in the azetidine derivative Ia by incorporating an appropriate spiro moiety. The cyclopropyl moiety was introduced by a diastereoselective rhodium catalyzed cyclopropanation reaction.
Keywords: Amino acids; carbenes; cyclopropanation; rhodium; spiro compounds.
Figures

Similar articles
-
Azetidinic amino acids: stereocontrolled synthesis and pharmacological characterization as ligands for glutamate receptors and transporters.Org Biomol Chem. 2005 Nov 7;3(21):3926-36. doi: 10.1039/b509514j. Epub 2005 Sep 29. Org Biomol Chem. 2005. PMID: 16240010
-
The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes.Pharmacol Ther. 2005 Sep;107(3):271-85. doi: 10.1016/j.pharmthera.2005.01.002. Epub 2005 Apr 14. Pharmacol Ther. 2005. PMID: 16112332 Review.
-
Synthesis and biology of the conformationally restricted ACPD analogue, 2-aminobicyclo[2.1.1]hexane-2,5-dicarboxylic acid-I, a potent mGluR agonist.J Med Chem. 1998 May 7;41(10):1641-50. doi: 10.1021/jm970719q. J Med Chem. 1998. PMID: 9572889
-
Synthesis, SARs, and pharmacological characterization of 2-amino-3 or 6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent, selective, and orally active group II metabotropic glutamate receptor agonists.J Med Chem. 2000 Dec 14;43(25):4893-909. doi: 10.1021/jm000346k. J Med Chem. 2000. PMID: 11123999
-
Glutamate receptors in the kidney.Nephrol Dial Transplant. 2015 Oct;30(10):1630-8. doi: 10.1093/ndt/gfv028. Epub 2015 Mar 31. Nephrol Dial Transplant. 2015. PMID: 25829324 Review.
References
-
- Meldrum B, editor. Excitatory Amino Acid Antagonists. Oxford, England: Blackwell; 1991.
-
- Collingridge G L, Watkins J C, editors. The NMDA Receptor. 2nd ed. Oxford, England: IRL Press; 1994.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
