Atherton-Todd reaction: mechanism, scope and applications
- PMID: 24991268
- PMCID: PMC4077366
- DOI: 10.3762/bjoc.10.117
Atherton-Todd reaction: mechanism, scope and applications
Abstract
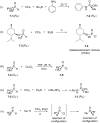
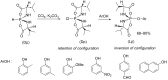
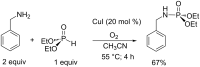
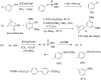
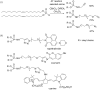
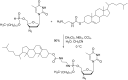
Initially, the Atherton-Todd (AT) reaction was applied for the synthesis of phosphoramidates by reacting dialkyl phosphite with a primary amine in the presence of carbon tetrachloride. These reaction conditions were subsequently modified with the aim to optimize them and the reaction was extended to different nucleophiles. The mechanism of this reaction led to controversial reports over the past years and is adequately discussed. We also present the scope of the AT reaction. Finally, we investigate the AT reaction by means of exemplary applications, which mainly concern three topics. First, we discuss the activation of a phenol group as a phosphate which allows for subsequent transformations such as cross coupling and reduction. Next, we examine the AT reaction applied to produce fire retardant compounds. In the last section, we investigate the use of the AT reaction for the production of compounds employed for biological applications. The selected examples to illustrate the applications of the Atherton-Todd reaction mainly cover the past 15 years.
Keywords: amphiphiles; flame retardant; lipid conjugates; organophosphorus; phosphate; phosphoramidate.
Figures
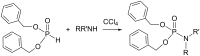



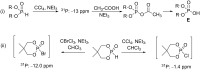
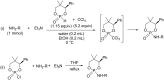

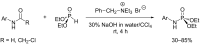
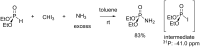

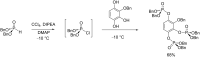






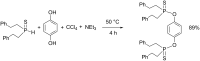



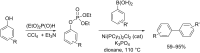








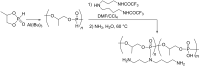

Similar articles
-
[Study on the phosphorylaions reaction between ethyl salicylate and DEPH].Guang Pu Xue Yu Guang Pu Fen Xi. 2004 Mar;24(3):379-80. Guang Pu Xue Yu Guang Pu Fen Xi. 2004. PMID: 15760006 Chinese.
-
A phosphoryl radical-initiated Atherton-Todd-type reaction under open air.Chem Commun (Camb). 2020 Jan 28;56(9):1357-1360. doi: 10.1039/c9cc09407e. Epub 2020 Jan 6. Chem Commun (Camb). 2020. PMID: 31904755
-
Synthesis of ortho-Phosphated (Hetero)Arylamines through Cascade Atherton-Todd Reaction/[3,3]-Rearrangement from Arylhydroxylamines and Dialkyl Phosphites.Org Lett. 2022 Oct 21;24(41):7690-7695. doi: 10.1021/acs.orglett.2c03269. Epub 2022 Oct 12. Org Lett. 2022. PMID: 36222849
-
Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications.Materials (Basel). 2017 Jul 11;10(7):784. doi: 10.3390/ma10070784. Materials (Basel). 2017. PMID: 28773147 Free PMC article. Review.
-
Opening up the Toolbox: Synthesis and Mechanisms of Phosphoramidates.Molecules. 2020 Aug 13;25(16):3684. doi: 10.3390/molecules25163684. Molecules. 2020. PMID: 32823507 Free PMC article. Review.
Cited by
-
Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water.Life (Basel). 2017 Jul 29;7(3):32. doi: 10.3390/life7030032. Life (Basel). 2017. PMID: 28758921 Free PMC article. Review.
-
Organophosphorus-catalyzed relay oxidation of H-Bpin: electrophilic C-H borylation of heteroarenes.Chem Sci. 2020 Nov 19;12(3):1031-1037. doi: 10.1039/d0sc05620k. Chem Sci. 2020. PMID: 34163869 Free PMC article.
-
Chlorine in an Organic Molecule, a Universal Promoter-Workhorse-Of Reactions.Molecules. 2023 Dec 5;28(24):7957. doi: 10.3390/molecules28247957. Molecules. 2023. PMID: 38138446 Free PMC article. Review.
-
Solid-Phase Synthesis of Phosphorothioate/Phosphonothioate and Phosphoramidate/Phosphonamidate Oligonucleotides.Molecules. 2019 May 15;24(10):1872. doi: 10.3390/molecules24101872. Molecules. 2019. PMID: 31096640 Free PMC article.
-
Chemoselective Activation of Diethyl Phosphonates: Modular Synthesis of Biologically Relevant Phosphonylated Scaffolds.Angew Chem Int Ed Engl. 2018 Oct 1;57(40):13330-13334. doi: 10.1002/anie.201806343. Epub 2018 Sep 11. Angew Chem Int Ed Engl. 2018. PMID: 30067301 Free PMC article.
References
-
- Atherton F R, Openshaw H T, Todd A R. J Chem Soc. 1945:660–663. doi: 10.1039/jr9450000660. - DOI
-
- Hasse G. Ber Dtsch Chem Ges. 1877;10:2185–2195. doi: 10.1002/cber.187701002245. - DOI
-
- Lippmann E, Fleissner F. Ber Dtsch Chem Ges. 1886;19:2467–2471. doi: 10.1002/cber.188601902188. - DOI
-
- Fuson R C, Bull B A. Chem Rev. 1934;15:275–309. doi: 10.1021/cr60052a001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
