Cyclization-endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst
- PMID: 24991279
- PMCID: PMC4077508
- DOI: 10.3762/bjoc.10.128
Cyclization-endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst
Abstract
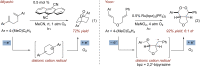
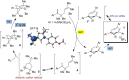
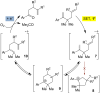
Triarylpyrylium salts were employed as single electron photooxidants to catalyze a cyclization-endoperoxidation cascade of dienes. The transformation is presumed to proceed via the intermediacy of diene cation radicals. The nature of the diene component was investigated in this context to determine the structural requirements necessary for successful reactivity. Several unique endoperoxide structures were synthesized in yields up to 79%.
Keywords: alkene; cascade; endoperoxide; oxidation; photoredox catalysis.
Figures



Similar articles
-
A General Approach to Catalytic Alkene Anti-Markovnikov Hydrofunctionalization Reactions via Acridinium Photoredox Catalysis.Acc Chem Res. 2016 Sep 20;49(9):1997-2006. doi: 10.1021/acs.accounts.6b00304. Epub 2016 Sep 2. Acc Chem Res. 2016. PMID: 27588818
-
Electrophilic Pt(II) complexes: precision instruments for the initiation of transformations mediated by the cation-olefin reaction.Acc Chem Res. 2014 Aug 19;47(8):2319-31. doi: 10.1021/ar500047j. Epub 2014 May 20. Acc Chem Res. 2014. PMID: 24845777 Free PMC article.
-
Merging Visible Light Photoredox and Gold Catalysis.Acc Chem Res. 2016 Oct 18;49(10):2261-2272. doi: 10.1021/acs.accounts.6b00351. Epub 2016 Sep 9. Acc Chem Res. 2016. PMID: 27610939
-
Difunctionalization of Dienes, Enynes and Related Compounds via Sequential Radical Addition and Cyclization Reactions.Molecules. 2023 Jan 23;28(3):1145. doi: 10.3390/molecules28031145. Molecules. 2023. PMID: 36770814 Free PMC article. Review.
-
Synthetic and mechanistic studies of the cycloisomerization and cyclization/hydrosilylation of functionalized dienes catalyzed by cationic palladium(II) complexes.Acc Chem Res. 2002 Oct;35(10):905-13. doi: 10.1021/ar010040n. Acc Chem Res. 2002. PMID: 12379143 Review.
Cited by
-
Structural elaboration of dicyanopyrazine: towards push-pull molecules with tailored photoredox activity.RSC Adv. 2019 Jul 31;9(41):23797-23809. doi: 10.1039/c9ra04731j. eCollection 2019 Jul 29. RSC Adv. 2019. PMID: 35530614 Free PMC article.
-
Guiding the Design of Organic Photocatalyst for PET-RAFT Polymerization: Halogenated Xanthene Dyes.Macromolecules. 2019 Jan 8;52(1):236-248. doi: 10.1021/acs.macromol.8b02517. Epub 2018 Dec 20. Macromolecules. 2019. PMID: 31537947 Free PMC article.
-
Photoinduced 1,2,3,4-tetrahydropyridine ring conversions.Beilstein J Org Chem. 2015 Nov 11;11:2166-70. doi: 10.3762/bjoc.11.234. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26664638 Free PMC article.
-
Investigating the Effect of Lewis Acid Co-catalysts on Photosensitized Visible-Light De Mayo Reactions.Org Lett. 2023 Jun 9;25(22):4098-4102. doi: 10.1021/acs.orglett.3c01321. Epub 2023 May 24. Org Lett. 2023. PMID: 37223948 Free PMC article.
-
Consecutive π-Lewis acidic metal-catalysed cyclisation/photochemical radical addition promoted by in situ generated 2-benzopyrylium as the photoredox catalyst.Chem Sci. 2024 Mar 19;15(16):6115-6121. doi: 10.1039/d4sc00808a. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665511 Free PMC article.
References
-
- Ando W, editor. Organic Peroxides. New York: John Wiley & Sons; 1992.
LinkOut - more resources
Full Text Sources
Other Literature Sources
