Asymmetric Ugi 3CR on isatin-derived ketimine: synthesis of chiral 3,3-disubstituted 3-aminooxindole derivatives
- PMID: 24991292
- PMCID: PMC4077467
- DOI: 10.3762/bjoc.10.141
Asymmetric Ugi 3CR on isatin-derived ketimine: synthesis of chiral 3,3-disubstituted 3-aminooxindole derivatives
Abstract
An efficient Ugi three-component reaction of a preformed chiral ketimine derived from isatin with various isonitrile and acid components has been developed. The reactions proceeded smoothly and in a stereocontrolled manner with regard to the new center of the Ugi products due to the stereoinduction of the amine chiral residue. A wide variety of novel chiral 3,3-disubstituted 3-aminooxindoles were obtained, a selection of which were subjected to post-Ugi transformations, paving the way to application as peptidomimetics.
Keywords: Ugi; isatin; multicomponent; oxindole; peptidomimetics.
Figures
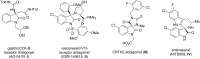

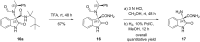
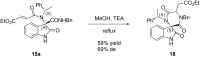
References
-
- Thakur P B, Sirisha K, Sarma A V S, Meshram H M. Tetrahedron Lett. 2014;55:2459–2462. doi: 10.1016/j.tetlet.2014.03.008. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
