A tandem Mannich addition-palladium catalyzed ring-closing route toward 4-substituted-3(2H)-furanones
- PMID: 24991301
- PMCID: PMC4077403
- DOI: 10.3762/bjoc.10.150
A tandem Mannich addition-palladium catalyzed ring-closing route toward 4-substituted-3(2H)-furanones
Abstract
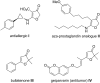
A facile route towards highly functionalized 3(2H)-furanones via a sequential Mannich addition-palladium catalyzed ring closing has been elaborated. The reaction of 4-chloroacetoacetate esters with imines derived from aliphatic and aromatic aldehydes under palladium catalysis afforded 4-substituted furanones in good to excellent yields. 4-Hydrazino-3(2H)-furanones could also be synthesized from diazo esters in excellent yields by utilising the developed strategy. We could also efficiently transform the substituted furanones to aza-prostaglandin analogues.
Keywords: 3(2H)-furanone; Mannich addition; aza-prostaglandin analogue; palladium catalysis; tandem reaction.
Figures




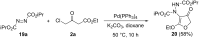

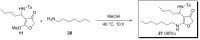
Similar articles
-
Palladium-Catalyzed Multicomponent Synthesis of Fully Substituted Alkylidene Furanones.Org Lett. 2020 Sep 4;22(17):7030-7033. doi: 10.1021/acs.orglett.0c02578. Epub 2020 Aug 26. Org Lett. 2020. PMID: 32846089
-
Reaction of polyhaloalkyl-substituted chromones, pyrones, and furanones with salicylaldehydes as a direct route to fused 2H-chromenes.J Org Chem. 2006 Jun 9;71(12):4538-43. doi: 10.1021/jo060459x. J Org Chem. 2006. PMID: 16749786
-
Cooperative Rh(II)/Pd(0) Dual Catalysis: Synthesis of Highly Substituted 3(2H)-Furanones with a C2-Quaternary Center via a Cyclization/Allylic Alkylation Cascade of α-Diazo-δ-keto-esters.Org Lett. 2021 Dec 3;23(23):9151-9156. doi: 10.1021/acs.orglett.1c03469. Epub 2021 Nov 15. Org Lett. 2021. PMID: 34780172
-
Palladium-catalyzed cross-coupling reactions of 4-tosyl-2(5H)-furanone with boronic acids: a facile and efficient route to generate 4-substituted 2(5H)-furanones.J Org Chem. 2003 Jan 24;68(2):670-3. doi: 10.1021/jo020640f. J Org Chem. 2003. PMID: 12530910
-
The naturally occurring furanones: formation and function from pheromone to food.Biol Rev Camb Philos Soc. 1999 Aug;74(3):259-76. doi: 10.1017/s0006323199005332. Biol Rev Camb Philos Soc. 1999. PMID: 10466251 Review.
References
-
- Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Oxford: Pergamon; 1992.
-
- Ogoshi S, Morimoto T, Nishio K, Ohe K, Murai S. J Org Chem. 1993;58:9–10. doi: 10.1021/jo00053a004. - DOI
-
- Ikeda I, Ohsuka A, Tani K, Hirao T, Kurosawa H. J Org Chem. 1996;61:4971–4974. doi: 10.1021/jo951425k. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
