A tandem Mannich addition-palladium catalyzed ring-closing route toward 4-substituted-3(2H)-furanones
- PMID: 24991301
- PMCID: PMC4077403
- DOI: 10.3762/bjoc.10.150
A tandem Mannich addition-palladium catalyzed ring-closing route toward 4-substituted-3(2H)-furanones
Abstract
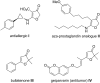
A facile route towards highly functionalized 3(2H)-furanones via a sequential Mannich addition-palladium catalyzed ring closing has been elaborated. The reaction of 4-chloroacetoacetate esters with imines derived from aliphatic and aromatic aldehydes under palladium catalysis afforded 4-substituted furanones in good to excellent yields. 4-Hydrazino-3(2H)-furanones could also be synthesized from diazo esters in excellent yields by utilising the developed strategy. We could also efficiently transform the substituted furanones to aza-prostaglandin analogues.
Keywords: 3(2H)-furanone; Mannich addition; aza-prostaglandin analogue; palladium catalysis; tandem reaction.
Figures




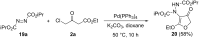

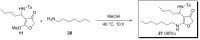
References
-
- Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Oxford: Pergamon; 1992.
-
- Ogoshi S, Morimoto T, Nishio K, Ohe K, Murai S. J Org Chem. 1993;58:9–10. doi: 10.1021/jo00053a004. - DOI
-
- Ikeda I, Ohsuka A, Tani K, Hirao T, Kurosawa H. J Org Chem. 1996;61:4971–4974. doi: 10.1021/jo951425k. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
