Direct dyes removal using modified magnetic ferrite nanoparticle
- PMID: 24991427
- PMCID: PMC4077710
- DOI: 10.1186/2052-336X-12-96
Direct dyes removal using modified magnetic ferrite nanoparticle
Abstract
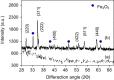
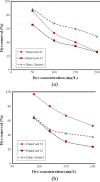
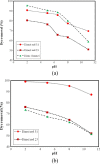
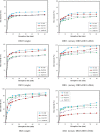
The magnetic adsorbent nanoparticle was modified using cationic surface active agent. Zinc ferrite nanoparticle and cetyl trimethylammonium bromide were used as an adsorbent and a surface active agent, respectively. Dye removal ability of the surface modified nanoparticle as an adsorbent was investigated. Direct Green 6 (DG6), Direct Red 31 (DR31) and Direct Red 23 (DR23) were used. The characteristics of the adsorbent were studied using Fourier transform infrared (FTIR), scanning electron microscopy (SEM) and X-ray diffraction (XRD). The effect of adsorbent dosage, initial dye concentration and salt was evaluated. In ternary system, dye removal of the adsorbent at 90, 120, 150 and 200 mg/L dye concentration was 63, 45, 30 and 23% for DR23, 97, 90, 78 and 45% for DR31 and 51, 48, 42 and 37% for DG6, respectively. It was found that dye adsorption onto the adsorbent followed Langmuir isotherm. The adsorption kinetic of dyes was found to conform to pseudo-second order kinetics.
Keywords: Adsorbent; Colored wastewater; Dye removal; Magnetic nanoparticle; Modification.
Figures




Similar articles
-
Gemini polymeric nanoarchitecture as a novel adsorbent: synthesis and dye removal from multicomponent system.J Colloid Interface Sci. 2013 Jun 15;400:88-96. doi: 10.1016/j.jcis.2013.03.014. Epub 2013 Mar 21. J Colloid Interface Sci. 2013. PMID: 23582906
-
Synthesis, characterization and dye removal ability of high capacity polymeric adsorbent: polyaminoimide homopolymer.J Hazard Mater. 2011 Dec 30;198:87-94. doi: 10.1016/j.jhazmat.2011.10.018. Epub 2011 Oct 8. J Hazard Mater. 2011. PMID: 22050931
-
Dye removal using modified copper ferrite nanoparticle and RSM analysis.Environ Monit Assess. 2013 Dec;185(12):10235-48. doi: 10.1007/s10661-013-3328-7. Epub 2013 Jul 13. Environ Monit Assess. 2013. PMID: 23852534
-
Separation of organic contaminant (dye) using the modified porous metal-organic framework (MIL).Environ Res. 2022 Nov;214(Pt 3):114006. doi: 10.1016/j.envres.2022.114006. Epub 2022 Aug 13. Environ Res. 2022. PMID: 35973465
-
Dye removal from water and wastewater by nanosized metal oxides - modified activated carbon: a review on recent researches.J Environ Health Sci Eng. 2020 Nov 5;18(2):1671-1689. doi: 10.1007/s40201-020-00566-w. eCollection 2020 Dec. J Environ Health Sci Eng. 2020. PMID: 33312670 Free PMC article. Review.
Cited by
-
Surface modification of MXene using cationic CTAB surfactant for adsorptive elimination of cefazolin antibiotic from water.Sci Rep. 2025 May 12;15(1):16416. doi: 10.1038/s41598-025-01435-y. Sci Rep. 2025. PMID: 40355521 Free PMC article.
-
Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions.Nanomaterials (Basel). 2021 Nov 19;11(11):3128. doi: 10.3390/nano11113128. Nanomaterials (Basel). 2021. PMID: 34835892 Free PMC article.
-
Simultaneous removal of Pb2+ and direct red 31 dye from contaminated water using N-(2-hydroxyethyl)-2-oxo-2H-chromene-3-carboxamide loaded chitosan nanoparticles.RSC Adv. 2022 Jun 29;12(29):18923-18935. doi: 10.1039/d2ra02526d. eCollection 2022 Jun 22. RSC Adv. 2022. PMID: 35873340 Free PMC article.
-
Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water.J Adv Res. 2017 Jul;8(4):435-443. doi: 10.1016/j.jare.2017.06.002. Epub 2017 Jun 10. J Adv Res. 2017. PMID: 28663825 Free PMC article.
-
Influence of preparation method on structural, optical, magnetic, and adsorption properties of nano-NiFe2O4.Environ Sci Pollut Res Int. 2019 Jul;26(21):21484-21494. doi: 10.1007/s11356-019-05498-z. Epub 2019 May 24. Environ Sci Pollut Res Int. 2019. PMID: 31127523
References
-
- Mahmoodi NM. Nickel ferrite nanoparticle: synthesis, modification by surfactant and dye removal ability. Water Air Soil Pollut. 2013;224(2):1–11.
-
- Ambashta RD, Sillanpää M. Water purification using magnetic assistance: a review. J Hazard Mater. 2010;180(1):38–49. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

