Glioblastoma multiforme: State of the art and future therapeutics
- PMID: 24991467
- PMCID: PMC4078454
- DOI: 10.4103/2152-7806.132138
Glioblastoma multiforme: State of the art and future therapeutics
Abstract
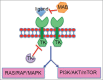
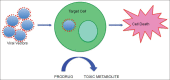
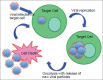
Background: Glioblastoma multiforme (GBM) is the most common and lethal primary malignancy of the central nervous system (CNS). Despite the proven benefit of surgical resection and aggressive treatment with chemo- and radiotherapy, the prognosis remains very poor. Recent advances of our understanding of the biology and pathophysiology of GBM have allowed the development of a wide array of novel therapeutic approaches, which have been developed. These novel approaches include molecularly targeted therapies, immunotherapies, and gene therapy.
Methods: We offer a brief review of the current standard of care, and a survey of novel therapeutic approaches for treatment of GBM.
Results: Despite promising results in preclinical trials, many of these therapies have demonstrated limited therapeutic efficacy in human clinical trials. Thus, although survival of patients with GBM continues to slowly improve, treatment of GBM remains extremely challenging.
Conclusion: Continued research and development of targeted therapies, based on a detailed understanding of molecular pathogenesis can reasonably be expected to yield improved outcomes for patients with GBM.
Keywords: Glioblastoma multiforme; gene therapy; immunotherapy; molecularly targeted therapy.
Figures

References
-
- Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: Overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26:825–53. - PubMed
-
- Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54:519–25. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

