Biocalcite, a multifunctional inorganic polymer: Building block for calcareous sponge spicules and bioseed for the synthesis of calcium phosphate-based bone
- PMID: 24991497
- PMCID: PMC4077312
- DOI: 10.3762/bjnano.5.72
Biocalcite, a multifunctional inorganic polymer: Building block for calcareous sponge spicules and bioseed for the synthesis of calcium phosphate-based bone
Abstract
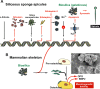
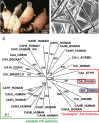
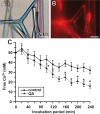
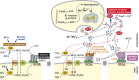
Calcium carbonate is the material that builds up the spicules of the calcareous sponges. Recent results revealed that the calcium carbonate/biocalcite-based spicular skeleton of these animals is formed through an enzymatic mechanism, such as the skeleton of the siliceous sponges, evolutionarily the oldest animals that consist of biosilica. The enzyme that mediates the calcium carbonate deposition has been identified as a carbonic anhydrase (CA) and has been cloned from the calcareous sponge species Sycon raphanus. Calcium carbonate deposits are also found in vertebrate bones besides the main constituent, calcium phosphate/hydroxyapatite (HA). Evidence has been presented that during the initial phase of HA synthesis poorly crystalline carbonated apatite is deposited. Recent data summarized here indicate that during early bone formation calcium carbonate deposits enzymatically formed by CA, act as potential bioseeds for the precipitation of calcium phosphate mineral onto bone-forming osteoblasts. Two different calcium carbonate phases have been found during CA-driven enzymatic calcium carbonate deposition in in vitro assays: calcite crystals and round-shaped vaterite deposits. The CA provides a new target of potential anabolic agents for treatment of bone diseases; a first CA activator stimulating the CA-driven calcium carbonate deposition has been identified. In addition, the CA-driven calcium carbonate crystal formation can be frozen at the vaterite state in the presence of silintaphin-2, an aspartic acid/glutamic acid-rich sponge-specific protein. The discovery that calcium carbonate crystals act as bioseeds in human bone formation may allow the development of novel biomimetic scaffolds for bone tissue engineering. Na-alginate hydrogels, enriched with biosilica, have recently been demonstrated as a suitable matrix to embed bone forming cells for rapid prototyping bioprinting/3D cell printing applications.
Keywords: biocalcite; bioprinting; bone; bone formation; calcareous spicules; sponge.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
