Enhancement of photocatalytic H2 evolution of eosin Y-sensitized reduced graphene oxide through a simple photoreaction
- PMID: 24991517
- PMCID: PMC4077509
- DOI: 10.3762/bjnano.5.92
Enhancement of photocatalytic H2 evolution of eosin Y-sensitized reduced graphene oxide through a simple photoreaction
Abstract
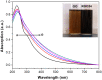
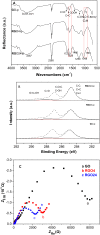
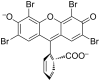
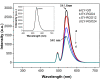
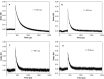
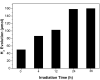
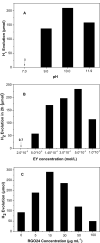
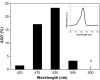
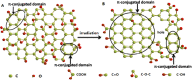
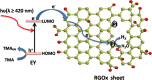
A graphene oxide (GO) solution was irradiated by a Xenon lamp to form reduced graphene oxide (RGO). After irradiation, the epoxy, the carbonyl and the hydroxy groups are gradually removed from GO, resulting in an increase of sp(2) π-conjugated domains and defect carbons with holes for the formed RGO. The RGO conductivity increases due to the restoration of sp(2) π-conjugated domains. The photocatalytic activity of EY-RGO/Pt for hydrogen evolution was investigated with eosin Y (EY) as a sensitizer of the RGO and Pt as a co-catalyst. When the irradiation time is increased from 0 to 24 h the activity rises, and then reaches a plateau. Under optimum conditions (pH 10.0, 5.0 × 10(-4) mol L(-1) EY, 10 μg mL(-1) RGO), the maximal apparent quantum yield (AQY) of EY-RGO24/Pt for hydrogen evolution rises up to 12.9% under visible light irradiation (λ ≥ 420 nm), and 23.4% under monochromatic light irradiation at 520 nm. Fluorescence spectra and transient absorption decay spectra of the EY-sensitized RGO confirm that the electron transfer ability of RGO increases with increasing irradiation time. The adsorption quantity of EY on the surface of RGO enhances, too. The two factors ultimately result in an enhancement of the photocatalytic hydrogen evolution over EY-RGO/Pt with increasing irradiation time. A possible mechanism is discussed.
Keywords: H2 evolution; eosin Y sensitization; graphene oxide; photocatalysis; photoreduction; sp2 conjugated domains.
Figures

Similar articles
-
Facile Synthesis of Graphene Sponge from Graphene Oxide for Efficient Dye-Sensitized H2 Evolution.ACS Appl Mater Interfaces. 2016 Jun 22;8(24):15187-95. doi: 10.1021/acsami.6b01805. Epub 2016 Jun 7. ACS Appl Mater Interfaces. 2016. PMID: 27244655
-
Peptide Self-Assembled Biofilm with Unique Electron Transfer Flexibility for Highly Efficient Visible-Light-Driven Photocatalysis.ACS Nano. 2015 Nov 24;9(11):11258-65. doi: 10.1021/acsnano.5b04884. Epub 2015 Oct 26. ACS Nano. 2015. PMID: 26473307
-
Modification of TiO2 with sulfate and phosphate for enhanced eosin Y-sensitized hydrogen evolution under visible light illumination.Photochem Photobiol Sci. 2013 Oct;12(10):1903-10. doi: 10.1039/c3pp50167a. Photochem Photobiol Sci. 2013. PMID: 23995340
-
Photo-induced synthesis of ternary Pt/rGO/COF photocatalyst with Pt nanoparticles precisely anchored on rGO for efficient visible-light-driven H2 evolution.J Colloid Interface Sci. 2022 Feb 15;608(Pt 3):2613-2622. doi: 10.1016/j.jcis.2021.10.183. Epub 2021 Nov 1. J Colloid Interface Sci. 2022. PMID: 34772502
-
Eosin Y-sensitized graphitic carbon nitride fabricated by heating urea for visible light photocatalytic hydrogen evolution: the effect of the pyrolysis temperature of urea.Phys Chem Chem Phys. 2013 May 28;15(20):7657-65. doi: 10.1039/c3cp44687e. Epub 2013 Apr 17. Phys Chem Chem Phys. 2013. PMID: 23591628
Cited by
-
Synthesis and biological activity evaluation of novel peroxo-bridged derivatives as potential anti-hepatitis B virus agents.Medchemcomm. 2016 Oct 19;8(1):148-151. doi: 10.1039/c6md00344c. eCollection 2017 Jan 1. Medchemcomm. 2016. PMID: 30108700 Free PMC article.
-
Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization.Sci Rep. 2015 Jun 12;5:10589. doi: 10.1038/srep10589. Sci Rep. 2015. PMID: 26068540 Free PMC article.
-
PtNi Alloy Cocatalyst Modification of Eosin Y-Sensitized g-C3N4/GO Hybrid for Efficient Visible-Light Photocatalytic Hydrogen Evolution.Nanoscale Res Lett. 2018 Feb 2;13(1):33. doi: 10.1186/s11671-018-2448-y. Nanoscale Res Lett. 2018. PMID: 29396656 Free PMC article.
-
A Series of Oleanolic Acid Derivatives as Anti-Hepatitis B Virus Agents: Design, Synthesis, and in Vitro and in Vivo Biological Evaluation.Molecules. 2016 Mar 24;21(4):402. doi: 10.3390/molecules21040402. Molecules. 2016. PMID: 27023498 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
