Remifentanil for endotracheal intubation in premature infants: A randomized controlled trial
- PMID: 24991608
- PMCID: PMC4076902
- DOI: 10.4103/2279-042X.117387
Remifentanil for endotracheal intubation in premature infants: A randomized controlled trial
Abstract
Objective: Endotracheal intubation is a common procedure in neonatal care. The objective of this study was to determine whether the premedication with remifentanil before intubation has analgesic effects in newborn infants.
Methods: A total of 40 premature infants who needed endotracheal intubation for intubation-surfactant-extubation method were randomly assigned in two groups of an equal number at two university hospitals. The control group was given 10 μg/kg atropine IV infusions in 1 min and then 2 ml normal saline. In the case group, the atropine was given with the same method and then remifentanil was administered 2 μg/kg IV infusions in 2 min.
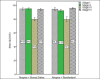
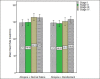
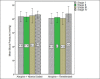
Findings: For remifentanil and control groups, the mean birth weight were 1761 ± 64 and 1447 ± 63 grams (P = 0.29), and the mean gestational ages were 31.69 ± 3.5 and 30.56 ± 2.8 weeks (P = 0.28), respectively. Using premature infant pain profile score, infants who received remifentanil felt significantly less pain than the control group (15.1 ± 1.6 vs. 7.5 ± 1.4; P < 0.001). There were no significant differences in the duration of endotracheal intubation procedure (20.8 ± 6 vs. 22.8 ± 7.3 s; P = 0.33), the number of attempts for successful intubation and oxygen desaturation between groups.
Conclusion: Premedication with remifentanil has good analgesic effects for endotracheal intubation in premature infants without significant derangements in mean blood pressure and oxygen saturation.
Keywords: Endotracheal intubation; Remifentanil; premature infant pain profile score; premedication.
Conflict of interest statement
Figures
Similar articles
-
Premedication with intravenous midazolam for neonatal endotracheal intubation: A double blind randomized controlled trial.J Res Med Sci. 2021 Aug 30;26:57. doi: 10.4103/jrms.JRMS_546_19. eCollection 2021. J Res Med Sci. 2021. PMID: 34729065 Free PMC article.
-
Remifentanil versus morphine-midazolam premedication on the quality of endotracheal intubation in neonates: a noninferiority randomized trial.J Pediatr. 2014 May;164(5):1032-7. doi: 10.1016/j.jpeds.2014.01.030. Epub 2014 Feb 25. J Pediatr. 2014. PMID: 24582007 Clinical Trial.
-
Remifentanil for endotracheal intubation in neonates: a randomised controlled trial.Arch Dis Child Fetal Neonatal Ed. 2010 Mar;95(2):F80-4. doi: 10.1136/adc.2009.167338. Arch Dis Child Fetal Neonatal Ed. 2010. PMID: 20231228 Clinical Trial.
-
Sedation of newborn infants for the INSURE procedure, are we sure?Biomed Res Int. 2013;2013:892974. doi: 10.1155/2013/892974. Epub 2013 Dec 23. Biomed Res Int. 2013. PMID: 24455736 Free PMC article.
-
Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation.Acta Paediatr. 2019 Feb;108(2):208-217. doi: 10.1111/apa.14606. Epub 2018 Dec 6. Acta Paediatr. 2019. PMID: 30290021 Review.
Cited by
-
Efficacy and Safety Aspects of Remifentanil Sedation for Intubation in Neonates: A Retrospective Study.Front Pediatr. 2019 Nov 7;7:450. doi: 10.3389/fped.2019.00450. eCollection 2019. Front Pediatr. 2019. PMID: 31788457 Free PMC article.
-
Efficacy, Safety, and Usability of Remifentanil as Premedication for INSURE in Preterm Neonates.Children (Basel). 2018 May 22;5(5):63. doi: 10.3390/children5050063. Children (Basel). 2018. PMID: 29789465 Free PMC article.
-
Remifentanil: applications in neonates.J Anesth. 2016 Jun;30(3):449-60. doi: 10.1007/s00540-015-2134-5. Epub 2016 Jan 13. J Anesth. 2016. PMID: 26758072 Review.
-
Premedication with intravenous midazolam for neonatal endotracheal intubation: A double blind randomized controlled trial.J Res Med Sci. 2021 Aug 30;26:57. doi: 10.4103/jrms.JRMS_546_19. eCollection 2021. J Res Med Sci. 2021. PMID: 34729065 Free PMC article.
-
Fetal, Preterm, and Term Neonate Exposure to Remifentanil: A Systematic Review of Efficacy and Safety.Paediatr Drugs. 2023 Sep;25(5):537-555. doi: 10.1007/s40272-023-00583-w. Epub 2023 Aug 4. Paediatr Drugs. 2023. PMID: 37541994
References
-
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2010 update. Neonatology. 2010;97:402–17. - PubMed
-
- Soll RF. Current trials in the treatment of respiratory failure in preterm infants. Neonatology. 2009;95:368–72. - PubMed
-
- Victorin LH, Deverajan LV, Curstedt T, Robertson B. Surfactant replacement in spontaneously breathing babies with hyaline membrane disease – A pilot study. Biol Neonate. 1990;58:121–6. - PubMed
-
- Duncan HP, Zurick NJ, Wolf AR. Should we reconsider awake neonatal intubation? A review of the evidence and treatment strategies. Paediatr Anaesth. 2001;11:135–45. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical