Cervical spondylotic myelopathy surgical trial: randomized, controlled trial design and rationale
- PMID: 24991714
- PMCID: PMC4633023
- DOI: 10.1227/NEU.0000000000000479
Cervical spondylotic myelopathy surgical trial: randomized, controlled trial design and rationale
Abstract
Background: Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in the world. There are significant practice variation and uncertainty as to the optimal surgical approach for treating CSM.
Objective: To determine whether ventral surgery is associated with superior Short Form-36 Physical Component Summary outcome at the 1-year follow-up compared with dorsal (laminectomy/fusion or laminoplasty) surgery for the treatment of CSM, to investigate whether postoperative sagittal balance is an independent predictor of overall outcome, and to compare health resource use for ventral and dorsal procedures.
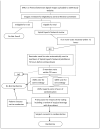
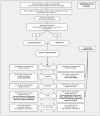
Methods: The study is a randomized, controlled trial with a nonrandomized arm for patients who are eligible but decline randomization. Two hundred fifty patients (159 randomized) with CSM from 11 sites will be recruited over 18 months. The primary outcome is the Short Form-36 Physical Component Summary score. Secondary outcomes include disease-specific outcomes, overall health-related quality of life (EuroQOL 5-dimension questionnaire), and health resource use.
Expected outcomes: This will be the first randomized, controlled trial to compare directly the health-related quality-of-life outcomes for ventral vs dorsal surgery for treating CSM.
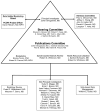
Discussion: A National Institutes of Health-funded (1R13AR065834-01) investigator meeting was held before the initiation of the trial to bring multiple stakeholders together to finalize the study protocol. Study investigators, coordinators, and major stakeholders were able to attend and discuss strengths of, limitations of, and concerns about the study. The final protocol was approved for funding by the Patient-Centered Outcomes Research Institute (CE-1304-6173). The trial began enrollment on April 1, 2014.
Trial registration: ClinicalTrials.gov NCT02076113.
Figures







Similar articles
-
Comparing Surgical Treatments for Cervical Spondylotic Myelopathy—The CSM-S Trial [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Dec. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Dec. PMID: 37703389 Free Books & Documents. Review.
-
Comparative effectiveness of ventral vs dorsal surgery for cervical spondylotic myelopathy.Neurosurgery. 2011 Mar;68(3):622-30; discussion 630-1. doi: 10.1227/NEU.0b013e31820777cf. Neurosurgery. 2011. PMID: 21164373 Clinical Trial.
-
Quality of life outcomes following cervical decompression for coexisting Parkinson's disease and cervical spondylotic myelopathy.Spine J. 2016 Nov;16(11):1358-1366. doi: 10.1016/j.spinee.2016.07.530. Epub 2016 Aug 2. Spine J. 2016. PMID: 27496286
-
A Comparative Study of Anterior Decompression With Fusion and Posterior Decompression With Laminoplasty for the Treatment of Cervical Spondylotic Myelopathy Patients With Large Anterior Compression of the Spinal Cord.Clin Spine Surg. 2017 Oct;30(8):E1137-E1142. doi: 10.1097/BSD.0000000000000500. Clin Spine Surg. 2017. PMID: 28099187
-
An Evidence-Based Stepwise Surgical Approach to Cervical Spondylotic Myelopathy: A Narrative Review of the Current Literature.World Neurosurg. 2016 Oct;94:97-110. doi: 10.1016/j.wneu.2016.06.109. Epub 2016 Jul 5. World Neurosurg. 2016. PMID: 27389939 Review.
Cited by
-
Patients May Return to Work Sooner After Laminoplasty: Occupational Outcomes of the Cervical Spondylotic Myelopathy Surgical Trial.Neurosurgery. 2025 Jan 1;96(1):131-141. doi: 10.1227/neu.0000000000003048. Epub 2024 Jun 24. Neurosurgery. 2025. PMID: 38912784 Free PMC article. Clinical Trial.
-
Degenerative Cervical Myelopathy; A Review of the Latest Advances and Future Directions in Management.Neurospine. 2019 Sep;16(3):494-505. doi: 10.14245/ns.1938314.157. Epub 2019 Aug 26. Neurospine. 2019. PMID: 31476852 Free PMC article.
-
Factors Influencing Surgical Decision-Making in the Posterior Laminectomy With Fixation for Degenerative Cervical Myelopathy (POLYFIX-DCM) Trial: Survey Study.JMIR Form Res. 2023 Sep 12;7:e48321. doi: 10.2196/48321. JMIR Form Res. 2023. PMID: 37698903 Free PMC article.
-
Socioeconomic and regional differences in the treatment of cervical spondylotic myelopathy.Surg Neurol Int. 2017 May 26;8:92. doi: 10.4103/sni.sni_471_16. eCollection 2017. Surg Neurol Int. 2017. PMID: 28607826 Free PMC article.
-
Change in Functional Impairment, Disability, and Quality of Life Following Operative Treatment for Degenerative Cervical Myelopathy: A Systematic Review and Meta-Analysis.Global Spine J. 2017 Sep;7(3 Suppl):53S-69S. doi: 10.1177/2192568217710137. Epub 2017 Sep 5. Global Spine J. 2017. PMID: 29164033 Free PMC article. Review.
References
-
- Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. American family physician. 2000 Sep 1;62(5):1064–1070. 1073. - PubMed
-
- Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005 May;56(5):1101–1113. discussion 1101-1113. - PubMed
-
- al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. Journal of neurosurgery. 1993 Oct;79(4):550–561. - PubMed
-
- Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007 Jan;60(1 Supp1 1):S35–41. - PubMed
-
- Rowland LP. Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology. 1992 Jan;42(1):5–13. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

