Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease
- PMID: 24991723
- PMCID: PMC10728583
- DOI: 10.1002/14651858.CD005055.pub3
Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease
Abstract
Background: Nitric oxide (NO) is a prevalent molecule in humans that is responsible for many physiologic activities including pulmonary vasodilation. An exogenous, inhaled form (iNO) exists that mimics this action without affecting systemic blood pressure. This therapy has been implemented in the treatment of pulmonary hypertension. This review examines the efficacy of iNO in the postoperative management of infants and children with congenital heart disease (CHD). The original review was published in 2005, updated in 2008 and again in 2014.
Objectives: To compare the effects of postoperative administration of iNO versus placebo or conventional management, or both, on infants and children with CHD and pulmonary hypertension. The primary outcome was mortality. Secondary outcomes included length of hospital stay; neurodevelopmental disability; number of pulmonary hypertensive crises (PHTC); changes in mean pulmonary arterial pressure (MPAP), mean arterial pressure (MAP), and heart rate (HR); changes in oxygenation measured as the ratio of arterial oxygen tension (PaO2) to fraction of inspired oxygen (FiO2); and measurement of maximum methaemoglobin level as a marker of toxicity.
Search methods: In this updated version we extended the CENTRAL search to 2013, Issue 12 of The Cochrane Library, and MEDLINE and EMBASE through to 1 December 2013. The original search was performed in July 2004 and again in November 2007. We included abstracts and all languages.
Selection criteria: We included randomized and quasi-randomized controlled trials comparing iNO with placebo or conventional management, or both. Trials included only children with CHD requiring surgery complicated by pulmonary hypertension.
Data collection and analysis: Two authors extracted data. Data were collected on mortality; number of PHTC; changes in MPAP, MAP, HR, and PaO2:FiO2; and maximum methaemoglobin level. Data on long-term mortality, neurodevelopmental disability, and length of hospital stay were unavailable. We performed subgroup analysis by method of control (placebo or conventional management).
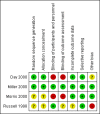
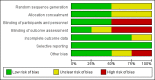
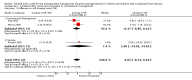
Main results: We reran the searches to December 2013 and identified three new studies. These three studies did not fulfil our inclusion criteria. Therefore, no new studies were included in this updated review. In total four randomized trials involving 210 participants were included in this review. We observed no differences in mortality (OR 1.67, 95% CI 0.38 to 7.30; P = 0.50); PHTC (OR 0.80, 95% CI 0.15 to 4.18; P = 0.79); changes in MPAP (treatment effect -2.94 mm Hg, 95% CI -9.28 to 3.40; P = 0.36), MAP (treatment effect -3.55 mm Hg, 95% CI -11.86 to 4.76; P = 0.40), HR (treatment effect 0.02 bpm, 95% CI -8.13 to 8.18; P = 1.00), or PaO2:FiO2 (mean difference 17.18, 95% CI -28.21 to 62.57; P = 0.46). There was a significant increase in the methaemoglobin level (mean difference 0.30%, 95% CI 0.24 to 0.36; P < 0.00001) in patients treated with iNO, although levels did not reach toxicity levels. Data from long-term mortality, neurodevelopmental disability, and length of stay were not available. Two trials had a low risk of bias. Very low quality of the evidence was observed considering grading of the outcomes.
Authors' conclusions: We observed no differences with the use of iNO in the outcomes reviewed. No data were available for several clinical outcomes including long-term mortality and neurodevelopmental outcome. We found it difficult to draw valid conclusions given concerns regarding methodologic quality, sample size, and heterogeneity.
Conflict of interest statement
Matthew Bizzarro: none known
Ian Gross: none known
Fabiano T Barbos: none known
Figures







Update of
-
Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005055. doi: 10.1002/14651858.CD005055.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2014 Jul 03;(7):CD005055. doi: 10.1002/14651858.CD005055.pub3. PMID: 16235391 Updated.
Similar articles
-
Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005055. doi: 10.1002/14651858.CD005055.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2014 Jul 03;(7):CD005055. doi: 10.1002/14651858.CD005055.pub3. PMID: 16235391 Updated.
-
Nitric oxide for respiratory failure in infants born at or near term.Cochrane Database Syst Rev. 2017 Jan 5;1(1):CD000399. doi: 10.1002/14651858.CD000399.pub3. Cochrane Database Syst Rev. 2017. PMID: 28056166 Free PMC article.
-
Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults.Cochrane Database Syst Rev. 2016 Jun 27;2016(6):CD002787. doi: 10.1002/14651858.CD002787.pub3. Cochrane Database Syst Rev. 2016. PMID: 27347773 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Inhaled nitric oxide for respiratory failure in preterm infants.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000509. doi: 10.1002/14651858.CD000509.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2010 Dec 08;(12):CD000509. doi: 10.1002/14651858.CD000509.pub4. PMID: 17636641 Updated.
Cited by
-
Inhaled pulmonary vasodilators: a narrative review.Ann Transl Med. 2021 Apr;9(7):597. doi: 10.21037/atm-20-4895. Ann Transl Med. 2021. PMID: 33987295 Free PMC article. Review.
-
Quality assessment of systematic reviews regarding dental implant placement on diabetic patients: an overview of systematic reviews.Med Oral Patol Oral Cir Bucal. 2019 Jul 1;24(4):e483-e490. doi: 10.4317/medoral.22955. Med Oral Patol Oral Cir Bucal. 2019. PMID: 31232387 Free PMC article.
-
A review of the use of inhaled nitric oxide in the PICU at Red Cross War Memorial Children's Hospital, 2011-2015: A retrospective cohort study.South Afr J Crit Care. 2021 Aug 6;37(2):10.7196/SAJCC.2021.v37i2.416. doi: 10.7196/SAJCC.2021.v37i2.416. eCollection 2021. South Afr J Crit Care. 2021. PMID: 35493980 Free PMC article.
-
Comparison of inhaled nitric oxide with aerosolized prostacyclin or analogues for the postoperative management of pulmonary hypertension: a systematic review and meta-analysis.Ann Med. 2020 May-Jun;52(3-4):120-130. doi: 10.1080/07853890.2020.1746826. Epub 2020 Apr 2. Ann Med. 2020. PMID: 32204626 Free PMC article.
-
Potential and Limitations of Cochrane Reviews in Pediatric Cardiology: A Systematic Analysis.Pediatr Cardiol. 2017 Apr;38(4):719-733. doi: 10.1007/s00246-017-1572-2. Epub 2017 Feb 27. Pediatr Cardiol. 2017. PMID: 28239752 Review.
References
References to studies included in this review
Day 2000 {published and unpublished data}
-
- Day RW, Hawkins JA, McGough EC, Crezee KL, Orsmond GS. Randomized controlled study of inhaled nitric oxide after operation for congenital heart surgery. Annals of Thoracic Surgery 2000;69(6):1907‐13. [MEDLINE: ] - PubMed
Miller 2000 {published data only}
-
- Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double‐blind study. Lancet 2000;356(9240):1464‐9. [MEDLINE: ] - PubMed
Morris 2000 {published data only}
-
- Morris K, Beghetti M, Petros A, Adatia I, Bohn D. Comparison of hyperventilation and inhaled nitric oxide for pulmonary hypertension after repair of congenital heart disease. Critical Care Medicine 2000;28(8):2974‐8. [MEDLINE: ] - PubMed
Russell 1998 {published data only}
-
- Russell IA, Zwass MS, Fineman JR, Balea M, Rouine‐Rapp K, Brook M, et al. The effects of inhaled nitric oxide on postoperative pulmonary hypertension in infants and children undergoing surgical repair of congenital heart disease. Anesthesia and Analgesia 1998;87(1):46‐51. - PubMed
References to studies excluded from this review
Argenziano 1998 {published data only}
-
- Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, et al. Randomized, double‐blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Annals of Thoracic Surgery 1998;65(2):340‐5. [MEDLINE: ] - PubMed
Checchia 2013 {published data only}
-
- Checchia PA, Bronicki RA, Muenzer JT, Dixon D, Raithel S, Gandhi SK, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children‐‐a randomized trial. Journal of Thoracic and Cardiovascular Surgery 2013;146(3):530‐6. [MEDLINE: ] - PubMed
Khazin 2004 {published data only}
-
- Khazin V, Kaufman Y, Zabeeda D, Medalion B, Sasson L, Schachner A, et al. Milrinone and nitric oxide: combined effect on pulmonary artery pressures after cardiopulmonary bypass in children. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(2):156‐9. [MEDLINE: ] - PubMed
Kirbas 2012 {published data only}
-
- Kirbas A, Yalcin Y, Tanrikulu N, Gürer O, Isik O. Comparison of inhaled nitric oxide and aerosolized iloprost in pulmonary hypertension in children with congenital heart surgery. Cardiology Journal 2012;19(4):387‐94. [MEDLINE: ] - PubMed
Loukhanov 2011 {published data only}
-
- Loukanov T, Bucsenez D, Springer W, Sebening C, Rauch H, Roesch E, et al. Comparison of inhaled nitric oxide with aerosolized iloprost for treatment of pulmonary hypertension in children after cardiopulmonary bypass surgery. Clinical Research in Cardiology 2011;100(7):595‐602. [MEDLINE: ] - PubMed
Matsui 1997 {published data only}
-
- Matsui J, Yahagi N, Kumon K, Hayashi H, Watanabe Y, Haruna M, et al. Effects of inhaled nitric oxide on postoperative pulmonary circulation in patients with congenital heart disease. Artificial Organs 1997;21(1):17‐20. [MEDLINE: ] - PubMed
Smith 2006 {published data only}
-
- Smith HA, Carter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. Journal of Thoracic and Cardiovascular Surgery 2006;132(1):58‐65. [MEDLINE: ] - PubMed
Additional references
Alderson 2002
-
- Alderson P, Green S, editors. Cochrane Collaboration open learning material for reviewers (Version 1.1, November 2002). www.cochrane‐net.org/openlearning/HTML/modA2‐3.htm.
Atz 1997
-
- Atz AM, Wessel DL. Inhaled nitric oxide in the neonate with cardiac disease. Seminars in Perinatology 1997;21(5):441‐55. [MEDLINE: ] - PubMed
Bando 1996
-
- Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, et al. Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. Journal of Thoracic and Cardiovascular Surgery 1996;112(6):1600‐9. [MEDLINE: ] - PubMed
Beghetti 1995
Clark 2000
-
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low‐dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. New England Journal of Medicine 2000;342(7):469‐74. [MEDLINE: ] - PubMed
CNRG 2004
-
- Cochrane Neonatal Review Group. Organization of a systematic review for the Cochrane Neonatal Review Group. Guidelines for reviewers and editors. www.cochrane.mcmaster.ca/neonatal/.
Curran 1995
-
- Curran RD, Mavroudis C, Backer CL, Sautel M, Zales VR, Wessel DL. Inhaled nitric oxide for children with congenital heart disease and pulmonary hypertension. Annals of Thoracic Surgery 1995;60(6):1765‐71. [MEDLINE: ] - PubMed
DiBlasi 2010
-
- DiBlasi RM, Myers TR, Hess DR. Evidence‐based clinical practice guideline: inhaled nitric oxide for neonates with acute hypoxic respiratory failure. Respiratory Care 2010;55(12):1717‐45. [MEDLINE: ] - PubMed
Dickersin 1994
Finer 2009
Gorenflo 2010
-
- Gorenflo M, Gu H, Xu Z. Peri‐operative pulmonary hypertension in paediatric patients: current strategies in children with congenital heart disease. Cardiology 2010;116(1):10‐7. [MEDLINE: ] - PubMed
Haworth 1984
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 1981
-
- Jones OD, Shore DF, Rigby ML, Leijala M, Scallan J, Shinebourne EA, et al. The use of tolazoline hydrochloride as a pulmonary vasodilator in potentially fatal episodes of pulmonary vasoconstriction after cardiac surgery in children. Circulation 1981;64(2 Part 2):II134‐9. [MEDLINE: ] - PubMed
Journois 1994
-
- Journois D, Pouard P, Mauriat P, Malhere T, Vouhe P, Safran D. Inhaled nitric oxide as a therapy for pulmonary hypertension after operations for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery 1994;107(4):1129‐35. [MEDLINE: ] - PubMed
Kinsella 1992
-
- Kinsella JP, Neish SR, Shaffer E, Abman SH. Low‐dose inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340(8823):819‐20. [MEDLINE: ] - PubMed
Lefebvre 1996
-
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Paper presented at the Fourth International Cochrane Colloquium. 20‐24 October, 1996.
Miller 1994
-
- Miller OI, Celermajer DS, Deanfield JE, Macrae DJ. Very low‐dose inhaled nitric oxide: a selective pulmonary vasodilator after operations for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery 1994;108(3):487‐94. [MEDLINE: ] - PubMed
Nelin 1998
-
- Nelin LD, Hoffman GM. The use of inhaled nitric oxide in a wide variety of clinical problems. Pediatric Clinics of North America 1998;45(3):531‐48. [MEDLINE: ] - PubMed
NINOS 1997
-
- Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full‐term and nearly full‐term infants with hypoxic respiratory failure. New England Journal of Medicine 1997;336(9):597‐604. [MEDLINE: ] - PubMed
RevMan 5.2
-
- RevMan 5.2. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 1992
-
- Roberts JD, Polaner DM, Lang P. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340(8823):818‐9. [MEDLINE: ] - PubMed
Roberts 1997
-
- Roberts JD, Fineman JR, Morin FC, Shawl PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. New England Journal of Medicine 1997;336(9):605‐10. [MEDLINE: ] - PubMed
Steudel 1999
-
- Steudel W, Hurford WE, Zapol WM. Inhaled nitric oxide. Basic biology and clinical applications. Anesthesiology 1999;91(4):1090‐121. [MEDLINE: ] - PubMed
Wessel 1993
-
- Wessel DL, Adatia I, Giglia TM, Thompson JE, Kulik TJ. Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation 1993;88 Suppl 1:2128‐38. [MEDLINE: ] - PubMed
Wheller 1979
-
- Wheller J, George BL, Mulder DG, Jarmakani JM. Diagnosis and management of postoperative pulmonary hypertensive crisis. Circulation 1979;60(7):1640‐4. [MEDLINE: ] - PubMed
Whittsett 1999
-
- Whittsett JA, Pryhuber GS, Rice WR, Warner BB, Wert SE. Acute respiratory disorders. In: Avery GB, Fletcher MA, MacDonald MG editor(s). Neonatology. Pathophysiology and Management of the Newborn. 5th Edition. Philadelphia: Lippincott, Williams, & Wilkins, 1999:499‐500.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

