Artefactual nanoparticle activation of the inflammasome platform: in vitro evidence with a nano-formed calcium phosphate
- PMID: 24991724
- PMCID: PMC4441531
- DOI: 10.2217/nnm.14.58
Artefactual nanoparticle activation of the inflammasome platform: in vitro evidence with a nano-formed calcium phosphate
Abstract
Aim: To determine whether in vitro experimental conditions dictate cellular activation of the inflammasome by apatitic calcium phosphate nanoparticles.
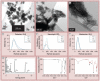
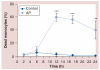
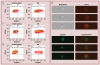
Material & methods: The responses of blood-derived primary human cells to in situ-formed apatite were investigated under different experimental conditions to assess the effect of aseptic culture, cell rest and duration of particle exposure. Cell death and particle uptake were assessed, while IL-1β and caspase 1 responses, with and without lipopolysaccharide prestimulation, were evaluated as markers of inflammasome activation.
Results: Under carefully addressed experimental conditions, apatitic nanoparticles did not induce cell death or engage the inflammasome platform, although both could be triggered through artefacts of experimentation.
Conclusion: In vitro studies often predict that engineered nanoparticles, such as synthetic apatite, are candidates for inflammasome activation and, hence, are toxic. However, the experimental setting must be very carefully considered as it may promote false-positive outcomes.
Keywords: IL-1β; apatite; caspase 1; experimental conditions; inflammasome; nanoparticle.
Figures



Similar articles
-
Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome.J Biol Chem. 2015 Feb 27;290(9):5926-39. doi: 10.1074/jbc.M114.610899. Epub 2015 Jan 15. J Biol Chem. 2015. PMID: 25593314 Free PMC article.
-
Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent.Toxicol Sci. 2016 Apr;150(2):473-87. doi: 10.1093/toxsci/kfw011. Epub 2016 Jan 21. Toxicol Sci. 2016. PMID: 26801583
-
Pro-inflammatory effects of human apatite crystals extracted from patients suffering from calcific tendinopathy.Arthritis Res Ther. 2021 Apr 29;23(1):131. doi: 10.1186/s13075-021-02516-9. Arthritis Res Ther. 2021. PMID: 33926523 Free PMC article.
-
The intersection of cell death and inflammasome activation.Cell Mol Life Sci. 2016 Jun;73(11-12):2349-67. doi: 10.1007/s00018-016-2205-2. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066895 Free PMC article. Review.
-
Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes.J Cell Physiol. 2019 May;234(5):5436-5450. doi: 10.1002/jcp.27475. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370619 Review.
Cited by
-
The formation and function of calciprotein particles.Pflugers Arch. 2025 Jun;477(6):753-772. doi: 10.1007/s00424-025-03083-7. Epub 2025 Apr 23. Pflugers Arch. 2025. PMID: 40266378 Free PMC article. Review.
-
The role of fetuin-A in mineral trafficking and deposition.Bonekey Rep. 2015 May 6;4:672. doi: 10.1038/bonekey.2015.39. eCollection 2015. Bonekey Rep. 2015. PMID: 25987986 Free PMC article. Review.
-
Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing.Part Fibre Toxicol. 2017 Dec 8;14(1):51. doi: 10.1186/s12989-017-0232-2. Part Fibre Toxicol. 2017. PMID: 29216926 Free PMC article.
-
Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity.Front Immunol. 2018 Sep 4;9:1991. doi: 10.3389/fimmu.2018.01991. eCollection 2018. Front Immunol. 2018. PMID: 30233585 Free PMC article.
-
Synthetic mimetics of the endogenous gastrointestinal nanomineral: Silent constructs that trap macromolecules for intracellular delivery.Nanomedicine. 2017 Feb;13(2):619-630. doi: 10.1016/j.nano.2016.07.008. Epub 2016 Jul 29. Nanomedicine. 2017. PMID: 27478107 Free PMC article.
References
-
- Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8(7):543–557. - PubMed
-
• Comprehensive review on the physicochemical factors that shape nanoparticle–cell interactions in biological surroundings.
-
- Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010;34(3):226–233. - PubMed
-
• Comprehensive review of the ‘bear traps’ in nanomineral characterization, definition and attribution of biological properties.
-
- Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7(12):779–786. - PubMed
-
• Emphasizes the importance of the particle corona in dictating its biological outcome.
-
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. - PubMed
-
• Seminal work that set the scene in particle and inflammasome research.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
