Ketamine alters cortical integration of GABAergic interneurons and induces long-term sex-dependent impairments in transgenic Gad67-GFP mice
- PMID: 24991763
- PMCID: PMC4123069
- DOI: 10.1038/cddis.2014.275
Ketamine alters cortical integration of GABAergic interneurons and induces long-term sex-dependent impairments in transgenic Gad67-GFP mice
Abstract
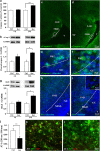
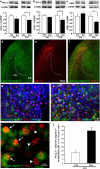
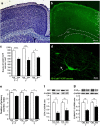
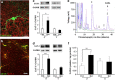
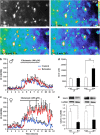
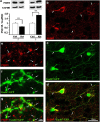
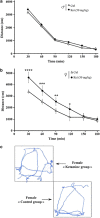
Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) antagonist, widely used as an anesthetic in neonatal pediatrics, is also an illicit drug named Super K or KitKat consumed by teens and young adults. In the immature brain, despite several studies indicating that NMDA antagonists are neuroprotective against excitotoxic injuries, there is more and more evidence indicating that these molecules exert a deleterious effect by suppressing a trophic function of glutamate. In the present study, we show using Gad67-GFP mice that prenatal exposure to ketamine during a time-window in which GABAergic precursors are migrating results in (i) strong apoptotic death in the ganglionic eminences and along the migratory routes of GABAergic interneurons; (ii) long-term deficits in interneuron density, dendrite numbers and spine morphology; (iii) a sex-dependent deregulation of γ-aminobutyric acid (GABA) levels and GABA transporter expression; (iv) sex-dependent changes in the response to glutamate-induced calcium mobilization; and (v) the long-term sex-dependent behavioral impairment of locomotor activity. In conclusion, using a preclinical approach, the present study shows that ketamine exposure during cortical maturation durably affects the integration of GABAergic interneurons by reducing their survival and differentiation. The resulting molecular, morphological and functional modifications are associated with sex-specific behavioral deficits in adults. In light of the present data, it appears that in humans, ketamine could be deleterious for the development of the brain of preterm neonates and fetuses of addicted pregnant women.
Figures

References
-
- Jégou S, El Ghazi F, de Lendeu PK, Marret S, Laudenbach V, Uguen A, et al. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann Neurol. 2012;72:952–960. - PubMed
-
- Cho GS, Lee JC, Ju C, Kim C, Kim WK. N-Methyl-D-aspartate receptor antagonists memantine and MK-801 attenuate the cerebral infarct accelerated by intracorpus callosum injection of lipopolysaccharides. Neurosci Lett. 2013;538:9–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

