Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma
- PMID: 24991839
- PMCID: PMC4203514
- DOI: 10.4161/auto.29118
Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma
Abstract
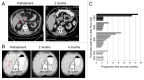
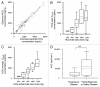
Blocking autophagy with hydroxychloroquine (HCQ) augments cell death associated with alkylating chemotherapy in preclinical models. This phase I study evaluated the maximum tolerated dose (MTD), safety, preliminary activity, pharmacokinetics, and pharmacodynamics of HCQ in combination with dose-intense temozolomide (TMZ) in patients with advanced solid malignancies. Forty patients (73% metastatic melanoma) were treated with oral HCQ 200 to 1200 mg daily with dose-intense oral TMZ 150 mg/m (2) daily for 7/14 d. This combination was well tolerated with no recurrent dose-limiting toxicities observed. An MTD was not reached for HCQ and the recommended phase II dose was HCQ 600 mg twice daily combined with dose-intense TMZ. Common toxicities included grade 2 fatigue (55%), anorexia (28%), nausea (48%), constipation (20%), and diarrhea (20%). Partial responses and stable disease were observed in 3/22 (14%) and 6/22 (27%) patients with metastatic melanoma. In the final dose cohort 2/6 patients with refractory BRAF wild-type melanoma had a near complete response, and prolonged stable disease, respectively. A significant accumulation in autophagic vacuoles (AV) in peripheral blood mononuclear cells was observed in response to combined therapy. Population pharmacokinetics (PK) modeling, individual PK simulations, and PK-pharmacodynamics (PD) analysis identified a threshold HCQ peak concentration that predicts therapy-associated AV accumulation. This study indicates that the combination of high-dose HCQ and dose-intense TMZ is safe and tolerable, and is associated with autophagy modulation in patients. Prolonged stable disease and responses suggest antitumor activity in melanoma patients, warranting further studies of this combination, or combinations of more potent autophagy inhibitors and chemotherapy in melanoma.
Keywords: autophagy; chemotherapy; clinical trial; hydroxychloroquine; melanoma.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Adonizio CS, Babb JS, Maiale C, Huang C, Donahue J, Millenson MM, Hosford M, Somer R, Treat J, Sherman E, et al. Temozolomide in non-small-cell lung cancer: preliminary results of a phase II trial in previously treated patients. Clin Lung Cancer. 2002;3:254–8. doi: 10.3816/CLC.2002.n.009. - DOI - PubMed
-
- Siena S, Crinò L, Danova M, Del Prete S, Cascinu S, Salvagni S, Schiavetto I, Vitali M, Bajetta E. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol. 2010;21:655–61. doi: 10.1093/annonc/mdp343. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
