Individualizing the WHO HIV and infant feeding guidelines: optimal breastfeeding duration to maximize infant HIV-free survival
- PMID: 24991902
- PMCID: PMC4098721
- DOI: 10.1097/QAD.0000000000000337
Individualizing the WHO HIV and infant feeding guidelines: optimal breastfeeding duration to maximize infant HIV-free survival
Abstract
Objectives: To determine how infant feeding recommendations can maximize HIV-free survival (HFS) among HIV-exposed, uninfected African infants, balancing risks of breast milk-associated HIV infection with setting-specific risks of illness and death associated with replacement feeding.
Design: Validated mathematical model of HIV-exposed, uninfected infants, with published data from Africa.
Methods: We projected 24-month HFS using combinations of: maternal CD4, antiretroviral drug availability, and relative risk of mortality among replacement-fed compared to breastfed infants ('RR-RF', range 1.0-6.0). For each combination, we identified the 'optimal' breastfeeding duration (0-24 months) maximizing HFS. We compared HFS under an 'individualized' approach, based on the above parameters, to the WHO 'public health approach' (12 months breastfeeding for all HIV-infected women).
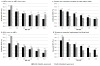
Results: Projected HFS was 65-93%. When the value of RR-RF is 1.0, replacement feeding from birth maximized HFS. At a commonly reported RR-RF value (2.0), optimal breastfeeding duration was 3-12 months, depending on maternal CD4 and antiretroviral drug availability. As the value of RR-RF increased, optimal breastfeeding duration increased. Compared to the public health approach, an individualized approach improved absolute HFS by less than 1% if RR-RF value was 2.0-4.0, by 3% if RR-RF value was 1.0 or 6.0, and by greater amounts if access to antiretroviral drugs was limited.
Conclusion: Tailoring breastfeeding duration to maternal CD4, antiretroviral drug availability, and local replacement feeding safety can optimize HFS among HIV-exposed infants. An individualized approach leads to moderate gains in HFS, but only when mortality risks from replacement feeding are very low or very high, or antiretroviral drug availability is limited. The WHO public health approach is beneficial in most resource-limited settings.
Conflict of interest statement
Figures

References
-
- World Health Organization. [Accessed February 25, 2014];Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010 at http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf. - PubMed
-
- Shapiro RL, Lockman S. Mortality among HIV-exposed infants: the first and final frontier. Clin Infect Dis. 2010;50:445–447. - PubMed
-
- Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. - PubMed
-
- Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- K24 AI062476/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UM1AI068616/AI/NIAID NIH HHS/United States
- R01 AI058736/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- N01-DK-9-001/DK/NIDDK NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1AI068632/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- K01 AI078754/AI/NIAID NIH HHS/United States
- R01 AI093269/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

