Tetherin can restrict cell-free and cell-cell transmission of HIV from primary macrophages to T cells
- PMID: 24991932
- PMCID: PMC4081785
- DOI: 10.1371/journal.ppat.1004189
Tetherin can restrict cell-free and cell-cell transmission of HIV from primary macrophages to T cells
Abstract
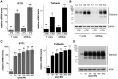
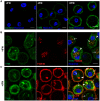
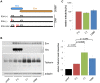
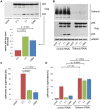
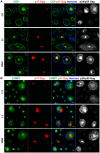
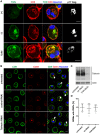
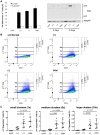
Bst-2/Tetherin inhibits the release of HIV by tethering newly formed virus particles to the plasma membrane of infected cells. Although the mechanisms of Tetherin-mediated restriction are increasingly well understood, the biological relevance of this restriction in the natural target cells of HIV is unclear. Moreover, whether Tetherin exerts any restriction on the direct cell-cell spread of HIV across intercellular contacts remains controversial. Here we analyse the restriction endogenous Tetherin imposes on HIV transmission from primary human macrophages, one of the main targets of HIV in vivo. We find that the mRNA and protein levels of Tetherin in macrophages are comparable to those in T cells from the same donors, and are highly upregulated by type I interferons. Improved immunocytochemistry protocols enable us to demonstrate that Tetherin localises to the cell surface, the trans-Golgi network, and the macrophage HIV assembly compartments. Tetherin retains budded virions in the assembly compartments, thereby impeding the release and cell-free spread of HIV, but it is not required for the maintenance of these compartments per se. Notably, using a novel assay to quantify cell-cell spread, we show that Tetherin promotes the transfer of virus clusters from macrophages to T cells and thereby restricts the direct transmission of a dual-tropic HIV-1. Kinetic analyses provide support for the notion that this direct macrophage-T cell spread is mediated, at least in part, by so-called virological synapses. Finally, we demonstrate that the viral Vpu protein efficiently downregulates the cell surface and overall levels of Tetherin, and thereby abrogates this HIV restriction in macrophages. Together, our study shows that Tetherin, one of the most potent HIV restriction factors identified to date, can inhibit virus spread from primary macrophages, regardless of the mode of transmission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Neil SJD, Zang T, Bieniasz PD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451: 425–430. - PubMed
-
- Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G (2007) Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci 120: 3850–3858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

