Activation and measurement of free whisking in the lightly anesthetized rodent
- PMID: 24992095
- PMCID: PMC4934662
- DOI: 10.1038/nprot.2014.119
Activation and measurement of free whisking in the lightly anesthetized rodent
Abstract
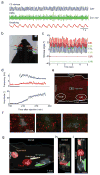
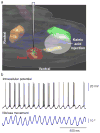
The rodent vibrissa system is a widely used experimental model of active sensation and motor control. Vibrissa-based touch in rodents involves stereotypic, rhythmic sweeping of the vibrissae as the animal explores its environment. Although pharmacologically induced rhythmic movements have long been used to understand the neural circuitry that underlies a variety of rhythmic behaviors, including locomotion, digestion and ingestion, these techniques have not been available for active sensory movements such as whisking. However, recent work that delineated the location of the central pattern generator for whisking has enabled pharmacological control over this behavior. Here we specify a protocol for the pharmacological induction of rhythmic vibrissa movements that mimic exploratory whisking. The rhythmic vibrissa movements are induced by local injection of a glutamatergic agonist, kainic acid. This protocol produces coordinated rhythmic vibrissa movements that are sustained for several hours in the anesthetized mouse or rat and thus provides unprecedented experimental control in studies related to vibrissa-based neuronal circuitry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking.J Neurophysiol. 2003 Nov;90(5):2950-63. doi: 10.1152/jn.00511.2003. Epub 2003 Aug 6. J Neurophysiol. 2003. PMID: 12904336
-
Anatomical loops and their electrical dynamics in relation to whisking by rat.Somatosens Mot Res. 1999;16(2):69-88. doi: 10.1080/08990229970528. Somatosens Mot Res. 1999. PMID: 10449057 Review.
-
Coherent electrical activity between vibrissa sensory areas of cerebellum and neocortex is enhanced during free whisking.J Neurophysiol. 2002 Apr;87(4):2137-48. doi: 10.1152/jn.00229.2001. J Neurophysiol. 2002. PMID: 11929931
-
Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat.J Neurophysiol. 2004 Sep;92(3):1700-7. doi: 10.1152/jn.00020.2004. Epub 2004 Apr 7. J Neurophysiol. 2004. PMID: 15331651
-
Circuits in the rodent brainstem that control whisking in concert with other orofacial motor actions.Neuroscience. 2018 Jan 1;368:152-170. doi: 10.1016/j.neuroscience.2017.08.034. Epub 2017 Aug 23. Neuroscience. 2018. PMID: 28843993 Free PMC article. Review.
Cited by
-
The Brainstem Oscillator for Whisking and the Case for Breathing as the Master Clock for Orofacial Motor Actions.Cold Spring Harb Symp Quant Biol. 2014;79:29-39. doi: 10.1101/sqb.2014.79.024794. Epub 2015 Apr 15. Cold Spring Harb Symp Quant Biol. 2014. PMID: 25876629 Free PMC article.
-
Theory of hierarchically organized neuronal oscillator dynamics that mediate rodent rhythmic whisking.Neuron. 2022 Nov 16;110(22):3833-3851.e22. doi: 10.1016/j.neuron.2022.08.020. Epub 2022 Sep 15. Neuron. 2022. PMID: 36113472 Free PMC article.
-
Circuits in the Ventral Medulla That Phase-Lock Motoneurons for Coordinated Sniffing and Whisking.Neural Plast. 2016;2016:7493048. doi: 10.1155/2016/7493048. Epub 2016 May 18. Neural Plast. 2016. PMID: 27293905 Free PMC article. Review.
-
Whisker-Mediated Touch System in Rodents: From Neuron to Behavior.Front Syst Neurosci. 2019 Aug 21;13:40. doi: 10.3389/fnsys.2019.00040. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31496942 Free PMC article. Review.
-
A change in behavioral state switches the pattern of motor output that underlies rhythmic head and orofacial movements.Curr Biol. 2023 May 22;33(10):1951-1966.e6. doi: 10.1016/j.cub.2023.04.008. Epub 2023 Apr 26. Curr Biol. 2023. PMID: 37105167 Free PMC article.
References
-
- Cullen KE. Sensory signals during active versus passive movement. Current Opinion in Neurobiology. 2004;14:698–706. - PubMed
-
- von Holst E. Relations between the central nervous system and the peripheral organ. British Journal of Animal Behavior. 1954;2:89–94.
-
- Vincent SB. The tactile hair of the white rat. Journal of Comparative Neurology. 1913;23:1–23.
-
- Rice FL, Fundin BT, Arvidsson J, Aldskogius H, Johansson O. Comprehensive immunofluoresce and lectin binding study of the innervation of vibrissae follicle sinus complexes on the mystacial pad of the rat. Journal of Comparative Neurology. 1997;385:149–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

