Brain-penetrant, orally bioavailable microtubule-stabilizing small molecules are potential candidate therapeutics for Alzheimer's disease and related tauopathies
- PMID: 24992153
- PMCID: PMC4111403
- DOI: 10.1021/jm5005623
Brain-penetrant, orally bioavailable microtubule-stabilizing small molecules are potential candidate therapeutics for Alzheimer's disease and related tauopathies
Abstract
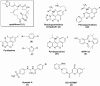
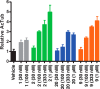
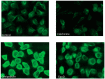
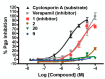
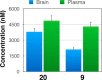
Microtubule (MT) stabilizing drugs hold promise as potential treatments for Alzheimer's disease (AD) and related tauopathies. However, thus far epothilone D has been the only brain-penetrant MT-stabilizer to be evaluated in tau transgenic mice and in AD patients. Furthermore, this natural product exhibits potential deficiencies as a drug candidate, including an intravenous route of administration and the inhibition of the P-glycoprotein (Pgp) transporter. Thus, the identification of alternative CNS-active MT-stabilizing agents that lack these potential limitations is of interest. Toward this objective, we have evaluated representative compounds from known classes of non-naturally occurring MT-stabilizing small molecules. This led to the identification of selected triazolopyrimidines and phenylpyrimidines that are orally bioavailable and brain-penetrant without disruption of Pgp function. Pharmacodynamic studies confirmed that representative compounds from these series enhance MT-stabilization in the brains of wild-type mice. Thus, these classes of MT-stabilizers hold promise for the development of orally active, CNS-directed MT-stabilizing therapies.
Figures

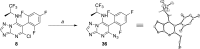



Similar articles
-
Characterization of Brain-Penetrant Pyrimidine-Containing Molecules with Differential Microtubule-Stabilizing Activities Developed as Potential Therapeutic Agents for Alzheimer's Disease and Related Tauopathies.J Pharmacol Exp Ther. 2016 May;357(2):432-50. doi: 10.1124/jpet.115.231175. Epub 2016 Mar 15. J Pharmacol Exp Ther. 2016. PMID: 26980057 Free PMC article.
-
The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies.Pharmacol Res. 2011 Apr;63(4):341-51. doi: 10.1016/j.phrs.2010.12.002. Epub 2010 Dec 14. Pharmacol Res. 2011. PMID: 21163349 Free PMC article.
-
The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice.J Neurosci. 2012 Mar 14;32(11):3601-11. doi: 10.1523/JNEUROSCI.4922-11.2012. J Neurosci. 2012. PMID: 22423084 Free PMC article.
-
Brain-penetrant microtubule-stabilizing compounds as potential therapeutic agents for tauopathies.Biochem Soc Trans. 2012 Aug;40(4):661-6. doi: 10.1042/BST20120010. Biochem Soc Trans. 2012. PMID: 22817712 Free PMC article. Review.
-
Microtubule stabilizing agents as potential treatment for Alzheimer's disease and related neurodegenerative tauopathies.J Med Chem. 2012 Nov 8;55(21):8979-96. doi: 10.1021/jm301079z. Epub 2012 Sep 28. J Med Chem. 2012. PMID: 23020671 Free PMC article. Review.
Cited by
-
Oxidative Aromatization of 4,7-Dihydro-6-nitroazolo[1,5-a]pyrimidines: Synthetic Possibilities and Limitations, Mechanism of Destruction, and the Theoretical and Experimental Substantiation.Molecules. 2021 Aug 4;26(16):4719. doi: 10.3390/molecules26164719. Molecules. 2021. PMID: 34443304 Free PMC article.
-
Brain-Penetrant Triazolopyrimidine and Phenylpyrimidine Microtubule Stabilizers as Potential Leads to Treat Human African Trypanosomiasis.ChemMedChem. 2018 Sep 6;13(17):1751-1754. doi: 10.1002/cmdc.201800404. Epub 2018 Aug 7. ChemMedChem. 2018. PMID: 29969537 Free PMC article.
-
Computational discovery of potential therapeutic agents against brain-eating amoeba (Naegleria fowleri).PLoS One. 2025 Jul 11;20(7):e0327621. doi: 10.1371/journal.pone.0327621. eCollection 2025. PLoS One. 2025. PMID: 40644400 Free PMC article.
-
Aminochrome Toxicity is Mediated by Inhibition of Microtubules Polymerization Through the Formation of Adducts with Tubulin.Neurotox Res. 2016 Apr;29(3):381-93. doi: 10.1007/s12640-015-9560-x. Epub 2015 Sep 7. Neurotox Res. 2016. PMID: 26345577
-
Region-specific dendritic simplification induced by Aβ, mediated by tau via dysregulation of microtubule dynamics: a mechanistic distinct event from other neurodegenerative processes.Mol Neurodegener. 2015 Nov 5;10:60. doi: 10.1186/s13024-015-0049-0. Mol Neurodegener. 2015. PMID: 26541821 Free PMC article.
References
-
- Ballatore C.; Lee V. M.-Y.; Trojanowski J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. - PubMed
-
- Buee L.; Bussiere T.; Buee-Scherrer V.; Delacourte A.; Hof P. R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130. - PubMed
-
- Roy S.; Zhang B.; Lee V. M.-Y.; Trojanowski J. Q. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005, 109, 5–13. - PubMed
-
- Brunden K. R.; Zhang B.; Carroll J.; Yao Y.; Potuzak J. S.; Hogan A. M.; Iba M.; James M. J.; Xie S. X.; Ballatore C.; Smith A. B.; Lee V. M.-Y.; Trojanowski J. Q. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 2010, 30, 13861–13866. - PMC - PubMed
-
- Zhang B.; Maiti A.; Shively S.; Lakhani F.; McDonald-Jones G.; Bruce J.; Lee E. B.; Xie S. X.; Joyce S.; Li C.; Toleikis P. M.; Lee V. M.-Y.; Trojanowski J. Q. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 227–231. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

