New aspects of the pathogenesis of canine distemper leukoencephalitis
- PMID: 24992230
- PMCID: PMC4113784
- DOI: 10.3390/v6072571
New aspects of the pathogenesis of canine distemper leukoencephalitis
Abstract
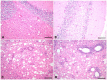
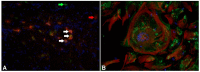
Canine distemper virus (CDV) is a member of the genus morbillivirus, which is known to cause a variety of disorders in dogs including demyelinating leukoencephalitis (CDV-DL). In recent years, substantial progress in understanding the pathogenetic mechanisms of CDV-DL has been made. In vivo and in vitro investigations provided new insights into its pathogenesis with special emphasis on axon-myelin-glia interaction, potential endogenous mechanisms of regeneration, and astroglial plasticity. CDV-DL is characterized by lesions with a variable degree of demyelination and mononuclear inflammation accompanied by a dysregulated orchestration of cytokines as well as matrix metalloproteinases and their inhibitors. Despite decades of research, several new aspects of the neuropathogenesis of CDV-DL have been described only recently. Early axonal damage seems to represent an initial and progressive lesion in CDV-DL, which interestingly precedes demyelination. Axonopathy may, thus, function as a potential trigger for subsequent disturbed axon-myelin-glia interactions. In particular, the detection of early axonal damage suggests that demyelination is at least in part a secondary event in CDV-DL, thus challenging the dogma of CDV as a purely primary demyelinating disease. Another unexpected finding refers to the appearance of p75 neurotrophin (NTR)-positive bipolar cells during CDV-DL. As p75NTR is a prototype marker for immature Schwann cells, this finding suggests that Schwann cell remyelination might represent a so far underestimated endogenous mechanism of regeneration, though this hypothesis still remains to be proven. Although it is well known that astrocytes represent the major target of CDV infection in CDV-DL, the detection of infected vimentin-positive astrocytes in chronic lesions indicates a crucial role of this cell population in nervous distemper. While glial fibrillary acidic protein represents the characteristic intermediate filament of mature astrocytes, expression of vimentin is generally restricted to immature or reactive astrocytes. Thus, vimentin-positive astrocytes might constitute an important cell population for CDV persistence and spread, as well as lesion progression. In vitro models, such as dissociated glial cell cultures, as well as organotypic brain slice cultures have contributed to a better insight into mechanisms of infection and certain morphological and molecular aspects of CDV-DL. Summarized, recent in vivo and in vitro studies revealed remarkable new aspects of nervous distemper. These new perceptions substantially improved our understanding of the pathogenesis of CDV-DL and might represent new starting points to develop novel treatment strategies.
Figures



References
-
- Deem S.L., Spelman L.H., Yates R.A., Montali R.J. Canine Distemper in Terrestrial Carnivores: A Review. J. Zoo Wildl. Med. 2000;31:441–451. - PubMed
-
- Greene C.E. Infectious Diseases of the Dog and Cat. 4th ed. Elsevier/Saunders; St. Louis, MO, USA: 2012. p. 1354.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

