Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes
- PMID: 24992257
- PMCID: PMC4081593
- DOI: 10.1371/journal.pone.0101629
Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes
Abstract
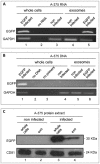
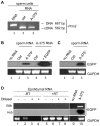
Mendelian laws provide the universal founding paradigm for the mechanism of genetic inheritance through which characters are segregated and assorted. In recent years, however, parallel with the rapid growth of epigenetic studies, cases of inheritance deviating from Mendelian patterns have emerged. Growing studies underscore phenotypic variations and increased risk of pathologies that are transgenerationally inherited in a non-Mendelian fashion in the absence of any classically identifiable mutation or predisposing genetic lesion in the genome of individuals who develop the disease. Non-Mendelian inheritance is most often transmitted through the germline in consequence of primary events occurring in somatic cells, implying soma-to-germline transmission of information. While studies of sperm cells suggest that epigenetic variations can potentially underlie phenotypic alterations across generations, no instance of transmission of DNA- or RNA-mediated information from somatic to germ cells has been reported as yet. To address these issues, we have now generated a mouse model xenografted with human melanoma cells stably expressing EGFP-encoding plasmid. We find that EGFP RNA is released from the xenografted human cells into the bloodstream and eventually in spermatozoa of the mice. Tumor-released EGFP RNA is associated with an extracellular fraction processed for exosome purification and expressing exosomal markers, in all steps of the process, from the xenografted cancer cells to the spermatozoa of the recipient animals, strongly suggesting that exosomes are the carriers of a flow of information from somatic cells to gametes. Together, these results indicate that somatic RNA is transferred to sperm cells, which can therefore act as the final recipients of somatic cell-derived information.
Conflict of interest statement
Figures


Similar articles
-
Bubbling beyond the barrier: exosomal RNA as a vehicle for soma-germline communication.J Physiol. 2024 Jun;602(11):2547-2563. doi: 10.1113/JP284420. Epub 2023 Nov 7. J Physiol. 2024. PMID: 37936475 Review.
-
Sperm-Mediated Transgenerational Inheritance.Front Microbiol. 2017 Dec 4;8:2401. doi: 10.3389/fmicb.2017.02401. eCollection 2017. Front Microbiol. 2017. PMID: 29255455 Free PMC article.
-
Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance.Mech Ageing Dev. 2017 Apr;163:15-22. doi: 10.1016/j.mad.2016.12.015. Epub 2017 Jan 16. Mech Ageing Dev. 2017. PMID: 28093237 Review.
-
Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance.J Theor Biol. 2014 Sep 21;357:143-9. doi: 10.1016/j.jtbi.2014.05.019. Epub 2014 May 22. J Theor Biol. 2014. PMID: 24859414
-
Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1.Mol Cancer. 2017 Aug 25;16(1):143. doi: 10.1186/s12943-017-0714-8. Mol Cancer. 2017. PMID: 28841829 Free PMC article.
Cited by
-
Heterogeneity in maternal mRNAs within clutches of eggs in response to thermal stress during the embryonic stage.BMC Ecol Evol. 2024 Feb 12;24(1):21. doi: 10.1186/s12862-024-02203-8. BMC Ecol Evol. 2024. PMID: 38347459 Free PMC article.
-
Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy.Int J Mol Sci. 2021 Feb 22;22(4):2165. doi: 10.3390/ijms22042165. Int J Mol Sci. 2021. PMID: 33671587 Free PMC article. Review.
-
Increased Plasmatic Levels of Exosomes Are Significantly Related to Relapse Rate in Patients with Oral Squamous Cell Carcinoma: A Cohort Study.Cancers (Basel). 2023 Dec 2;15(23):5693. doi: 10.3390/cancers15235693. Cancers (Basel). 2023. PMID: 38067397 Free PMC article.
-
Early satellite cell communication creates a permissive environment for long-term muscle growth.iScience. 2021 Mar 29;24(4):102372. doi: 10.1016/j.isci.2021.102372. eCollection 2021 Apr 23. iScience. 2021. PMID: 33948557 Free PMC article.
-
Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells.Front Oncol. 2019 May 3;9:356. doi: 10.3389/fonc.2019.00356. eCollection 2019. Front Oncol. 2019. PMID: 31131261 Free PMC article. Review.
References
-
- Bonduriansky R, Day T (2009) Nongenetic inheritance and its evolutionary implications. Annu Rev Ecol Evol Syst 40: 103–125.
-
- Sharma A (2012) Transgenerational epigenetic inheritance: focus on soma to germline information transfer. Prog Bioph Mol Biol doi: 10.1016/j.pbiomolbio. - PubMed
-
- Daxinger L, Whitelaw E (2012) Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nature Rev Gen 13: 153–162. Review. - PubMed
-
- Gluckman PD, Hanson MA, Beedle AS (2007) Non-genomic transgenerational inheritance of disease risk. BioEssays 29: 145–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

