Effects of ASKP1240 combined with tacrolimus or mycophenolate mofetil on renal allograft survival in Cynomolgus monkeys
- PMID: 24992357
- PMCID: PMC4175122
- DOI: 10.1097/TP.0000000000000236
Effects of ASKP1240 combined with tacrolimus or mycophenolate mofetil on renal allograft survival in Cynomolgus monkeys
Abstract
Background: Blocking the CD40-CD154 signal pathway has previously shown promise as a strategy to prevent allograft rejection. In this study, the efficacy of a novel fully human anti-CD40 monoclonal antibody-ASKP1240, administered as a monotherapy or combination therapy (subtherapeutic dose of tacrolimus or mycophenolate mofetil), on the prevention of renal allograft rejection was evaluated in Cynomolgus monkeys.
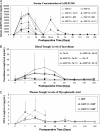
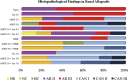
Methods: Heterotopic kidney transplants were performed in ABO-compatible, stimulation index 2.5 or higher in the two-way mixed lymphocyte reaction monkey pairs. Animals were divided into 12 groups and observed for a maximum of 180 days. Histopathologic, hematology, and biochemistry analyses were conducted in all groups. Cytokine release (interleukin [IL]-2, IL-4, IL-5, IL-6, tumor necrosis factor, and interferon-γ) was investigated in several groups.
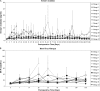
Results: ASKP1240 prolonged renal allograft survival in a dose-dependent manner in monotherapy. Low-dose (2 mg/kg) or high-dose (5 mg/kg) ASKP1240, in combination with mycophenolate mofetil (15 mg/kg) or tacrolimus (1 mg/kg), showed a significantly longer allograft survival time compared with monotherapy groups. No obvious side effects including drug-related thromboembolic complications were found. Cytokine release was not induced by ASKP1240 administration.
Conclusion: The present study indicates that ASKP1240, alone or in combination with other immunosuppressive drugs, could be a promising antirejection agent in organ transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates.Am J Transplant. 2012 Jul;12(7):1740-54. doi: 10.1111/j.1600-6143.2012.04014.x. Epub 2012 Mar 15. Am J Transplant. 2012. PMID: 22420525
-
Replacement of mycophenolate mofetil with a JAK inhibitor, AS2553627, in combination with low-dose tacrolimus, for renal allograft rejection in non-human primates.Int Immunopharmacol. 2018 Nov;64:201-207. doi: 10.1016/j.intimp.2018.08.029. Epub 2018 Sep 7. Int Immunopharmacol. 2018. PMID: 30195818
-
Long-term kidney allograft function and survival in prednisone-free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus.Clin J Am Soc Nephrol. 2012 Mar;7(3):504-12. doi: 10.2215/CJN.06940711. Epub 2012 Jan 26. Clin J Am Soc Nephrol. 2012. PMID: 22282478 Free PMC article. Clinical Trial.
-
Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option?Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
-
Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity.Pediatr Transplant. 2006 Sep;10(6):721-9. doi: 10.1111/j.1399-3046.2006.00577.x. Pediatr Transplant. 2006. PMID: 16911497 Review.
Cited by
-
CD11b is a novel alternate receptor for CD154 during alloimmunity.Am J Transplant. 2020 Aug;20(8):2216-2225. doi: 10.1111/ajt.15835. Epub 2020 Mar 30. Am J Transplant. 2020. PMID: 32149455 Free PMC article.
-
Costimulation Blockade in Kidney Transplant Recipients.Drugs. 2020 Jan;80(1):33-46. doi: 10.1007/s40265-019-01226-6. Drugs. 2020. PMID: 31749062 Free PMC article. Review.
-
Impact of infection on transplantation tolerance.Immunol Rev. 2019 Nov;292(1):243-263. doi: 10.1111/imr.12803. Epub 2019 Sep 19. Immunol Rev. 2019. PMID: 31538351 Free PMC article. Review.
-
Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches.Cell Mol Immunol. 2017 Feb;14(2):146-179. doi: 10.1038/cmi.2016.39. Epub 2016 Oct 10. Cell Mol Immunol. 2017. PMID: 27721455 Free PMC article. Review.
-
Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model.Xenotransplantation. 2015 May-Jun;22(3):221-30. doi: 10.1111/xen.12166. Epub 2015 Apr 3. Xenotransplantation. 2015. PMID: 25847130 Free PMC article.
References
-
- Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol 1988; 140: 3324. - PubMed
-
- Pype S, Declercq W, Ibrahimi A, et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem 2000; 275: 18586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

