Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration
- PMID: 24992450
- PMCID: PMC4080701
- DOI: 10.1038/srep05515
Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration
Abstract
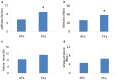
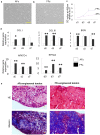
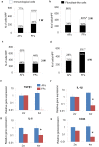
Injured adult tendons do not exhibit optimal healing through a regenerative process, whereas fetal tendons can heal in a regenerative fashion without scar formation. Hence, we compared FFs (mouse fetal fibroblasts) and AFs (mouse adult fibroblasts) as seed cells for the fabrication of scaffold-free engineered tendons. Our results demonstrated that FFs had more potential for tendon tissue engineering, as shown by higher levels of tendon-related gene expression. In the in situ AT injury model, the FFs group also demonstrated much better structural and functional properties after healing, with higher levels of collagen deposition and better microstructure repair. Moreover, fetal fibroblasts could increase the recruitment of fibroblast-like cells and reduce the infiltration of inflammatory cells to the injury site during the regeneration process. Our results suggest that the underlying mechanisms of better regeneration with FFs should be elucidated and be used to enhance adult tendon healing. This may assist in the development of future strategies to treat tendon injuries.
Figures





Similar articles
-
Transplantation of fetal instead of adult fibroblasts reduces the probability of ectopic ossification during tendon repair.Tissue Eng Part A. 2014 Jul;20(13-14):1815-26. doi: 10.1089/ten.TEA.2013.0296. Epub 2014 May 15. Tissue Eng Part A. 2014. PMID: 24410299 Free PMC article.
-
The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration.Biomaterials. 2010 Oct;31(28):7239-49. doi: 10.1016/j.biomaterials.2010.05.040. Biomaterials. 2010. PMID: 20615544
-
Type 2 diabetes impairs tendon repair after injury in a rat model.J Appl Physiol (1985). 2012 Dec 1;113(11):1784-91. doi: 10.1152/japplphysiol.00767.2012. Epub 2012 Oct 4. J Appl Physiol (1985). 2012. PMID: 23042903
-
Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm.Birth Defects Res C Embryo Today. 2013 Sep;99(3):203-222. doi: 10.1002/bdrc.21041. Birth Defects Res C Embryo Today. 2013. PMID: 24078497 Free PMC article. Review.
-
Is the tendon embryogenesis process resurrected during tendon healing?Plast Reconstr Surg. 2003 Sep;112(3):844-54. doi: 10.1097/01.PRS.0000070180.62037.FC. Plast Reconstr Surg. 2003. PMID: 12960868 Review.
Cited by
-
Understanding Fibrous Tissue in the Effective Healing of Rotator Cuff Injury.J Surg Res (Houst). 2024;7(2):215-228. doi: 10.26502/jsr.10020363. Epub 2024 May 21. J Surg Res (Houst). 2024. PMID: 38872898 Free PMC article.
-
Engineering Musculoskeletal Grafts for Multi-Tissue Unit Repair: Lessons From Developmental Biology and Wound Healing.Front Physiol. 2021 Aug 24;12:691954. doi: 10.3389/fphys.2021.691954. eCollection 2021. Front Physiol. 2021. PMID: 34504435 Free PMC article. Review.
-
Ascorbic Acid Promotes the Stemness of Corneal Epithelial Stem/Progenitor Cells and Accelerates Epithelial Wound Healing in the Cornea.Stem Cells Transl Med. 2017 May;6(5):1356-1365. doi: 10.1002/sctm.16-0441. Epub 2017 Mar 9. Stem Cells Transl Med. 2017. PMID: 28276172 Free PMC article.
-
MicroRNAs Associated with Shoulder Tendon Matrisome Disorganization in Glenohumeral Arthritis.PLoS One. 2016 Dec 16;11(12):e0168077. doi: 10.1371/journal.pone.0168077. eCollection 2016. PLoS One. 2016. PMID: 27992561 Free PMC article.
-
miRNA control of tissue repair and regeneration.Am J Pathol. 2015 Oct;185(10):2629-40. doi: 10.1016/j.ajpath.2015.04.001. Epub 2015 Jun 6. Am J Pathol. 2015. PMID: 26056933 Free PMC article. Review.
References
-
- Butler D. L., Juncosa N. & Dressler M. R. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng 6, 303–29 (2004). - PubMed
-
- Bach B. R. Jr et al. Single-incision endoscopic anterior cruciate ligament reconstruction using patellar tendon autograft. Minimum two-year follow-up evaluation. Am. J. Sports Med. 26, 30–40 (1998). - PubMed
-
- Bach B. R. Jr et al. Arthroscopically assisted anterior cruciate ligament reconstruction using patellar tendon autograft. Five- to nine-year follow-up evaluation. Am. J. Sports Med. 26, 20–9 (1998). - PubMed
-
- Badylak S. F. et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 29, 977–85 (1995). - PubMed
-
- Maletius W. & Gillquist J. Long-term results of anterior cruciate ligament reconstruction with a Dacron prosthesis. The frequency of osteoarthritis after seven to eleven years. Am. J. Sports Med. 25, 288–93 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

