Transplanting supersites of HIV-1 vulnerability
- PMID: 24992528
- PMCID: PMC4084637
- DOI: 10.1371/journal.pone.0099881
Transplanting supersites of HIV-1 vulnerability
Erratum in
- PLoS One. 2014;9(8); e105659. doi: 10.1371/journal.pone.0105659. Kwon, Peter D [corrected to Kwong, Peter D]
Abstract
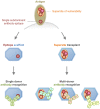
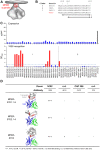
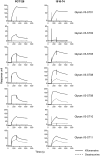
One strategy for isolating or eliciting antibodies against a specific target region on the envelope glycoprotein trimer (Env) of the human immunodeficiency virus type 1 (HIV-1) involves the creation of site transplants, which present the target region on a heterologous protein scaffold with preserved antibody-binding properties. If the target region is a supersite of HIV-1 vulnerability, recognized by a collection of broadly neutralizing antibodies, this strategy affords the creation of "supersite transplants", capable of binding (and potentially eliciting) antibodies similar to the template collection of effective antibodies. Here we transplant three supersites of HIV-1 vulnerability, each targeted by effective neutralizing antibodies from multiple donors. To implement our strategy, we chose a single representative antibody against each of the target supersites: antibody 10E8, which recognizes the membrane-proximal external region (MPER) on the HIV-1 gp41 glycoprotein; antibody PG9, which recognizes variable regions one and two (V1V2) on the HIV-1 gp120 glycoprotein; and antibody PGT128 which recognizes a glycopeptide supersite in variable region 3 (glycan V3) on gp120. We used a structural alignment algorithm to identify suitable acceptor proteins, and then designed, expressed, and tested antigenically over 100-supersite transplants in a 96-well microtiter-plate format. The majority of the supersite transplants failed to maintain the antigenic properties of their respective template supersite. However, seven of the glycan V3-supersite transplants exhibited nanomolar affinity to effective neutralizing antibodies from at least three donors and recapitulated the mannose9-N-linked glycan requirement of the template supersite. The binding of these transplants could be further enhanced by placement into self-assembling nanoparticles. Essential elements of the glycan V3 supersite, embodied by as few as 3 N-linked glycans and ∼ 25 Env residues, can be segregated into acceptor scaffolds away from the immune-evading capabilities of the rest of HIV-1 Env, thereby providing a means to focus the immune response on the scaffolded supersite.
Conflict of interest statement
Figures



References
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, et al. (2004) HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5: 233–236. - PubMed
-
- Starcich BR, Hahn BH, Shaw GM, McNeely PD, Modrow S, et al. (1986) Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45: 637–648. - PubMed
-
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, et al. (1998) The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393: 705–711. - PubMed
-
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, et al. (2002) HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420: 678–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

