Renalase: its role as a cytokine, and an update on its association with type 1 diabetes and ischemic stroke
- PMID: 24992568
- PMCID: PMC4383282
- DOI: 10.1097/MNH.0000000000000044
Renalase: its role as a cytokine, and an update on its association with type 1 diabetes and ischemic stroke
Abstract
Purpose of review: Remarkable progress has been achieved over the past 2 years in understanding the cellular actions of renalase, its pathophysiology and potential therapeutic utility.
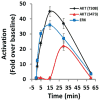
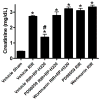
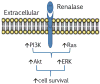
Recent findings: There has been a paradigm shift in our thinking about the mechanisms underlying the cellular actions of renalase. We now understand that, independent of its enzymatic properties, renalase functions as a signaling molecule, a cytokine that interacts with a yet-to-be identified plasma membrane receptor(s) to activate protein kinase B and the mitogen-activated protein kinase pathway. These signaling properties are critical to its cytoprotective effects. New information regarding renalase's enzymatic function as an α-nicotinamide adenine dinucleotide oxidase/anomerase will be reviewed. Lastly, we will discuss the association of certain single nucleotide polymorphisms in the renalase gene with type 1 diabetes and with ischemic stroke, and the clinical implications of these findings.
Summary: The consistent association of renalase single nucleotide polymorphisms and the development of type 1 diabetes is a great interest particularly because we now understand that renalase functions as a cytokine. Future work on renalase should focus on exploring the identity of its receptor(s), and its potential role as an immune modulator.
Conflict of interest statement
G.V.D. is a named inventor on several issued patents for the discovery and therapeutic utility of renalase.
Figures


Similar articles
-
Extracellular renalase protects cells and organs by outside-in signalling.J Cell Mol Med. 2017 Jul;21(7):1260-1265. doi: 10.1111/jcmm.13062. Epub 2017 Feb 26. J Cell Mol Med. 2017. PMID: 28238213 Free PMC article. Review.
-
Renalase-A new understanding of its enzymatic and non-enzymatic activity and its implications for future research.Clin Exp Pharmacol Physiol. 2022 Jan;49(1):3-9. doi: 10.1111/1440-1681.13594. Epub 2021 Oct 19. Clin Exp Pharmacol Physiol. 2022. PMID: 34545616 Review.
-
Identification of a receptor for extracellular renalase.PLoS One. 2015 Apr 23;10(4):e0122932. doi: 10.1371/journal.pone.0122932. eCollection 2015. PLoS One. 2015. PMID: 25906147 Free PMC article.
-
Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke.Neuromolecular Med. 2011 Dec;13(4):321-7. doi: 10.1007/s12017-011-8158-6. Epub 2011 Oct 2. Neuromolecular Med. 2011. PMID: 21964580 Free PMC article.
-
Renalase Challenges the Oxidative Stress and Fibroproliferative Response in COVID-19.Oxid Med Cell Longev. 2022 Sep 12;2022:4032704. doi: 10.1155/2022/4032704. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36132227 Free PMC article. Review.
Cited by
-
Renalase protects against contrast-induced nephropathy in Sprague-Dawley rats.PLoS One. 2015 Jan 30;10(1):e0116583. doi: 10.1371/journal.pone.0116583. eCollection 2015. PLoS One. 2015. PMID: 25635854 Free PMC article.
-
Limb ischemic preconditioning protects against contrast-induced nephropathy via renalase.EBioMedicine. 2016 Jul;9:356-365. doi: 10.1016/j.ebiom.2016.05.017. Epub 2016 May 18. EBioMedicine. 2016. PMID: 27333047 Free PMC article.
-
Renalase: A Multi-Functional Signaling Molecule with Roles in Gastrointestinal Disease.Cells. 2021 Aug 6;10(8):2006. doi: 10.3390/cells10082006. Cells. 2021. PMID: 34440775 Free PMC article. Review.
-
Polymorphism of the renalase gene in gestational diabetes mellitus.Endocrine. 2017 Jan;55(1):124-129. doi: 10.1007/s12020-016-1058-7. Epub 2016 Aug 9. Endocrine. 2017. PMID: 27507673
-
Renalase: a novel regulator of cardiometabolic and renal diseases.Hypertens Res. 2022 Oct;45(10):1582-1598. doi: 10.1038/s41440-022-00986-1. Epub 2022 Aug 8. Hypertens Res. 2022. PMID: 35941358 Free PMC article. Review.
References
-
- Li G, Xu J, Wang P, et al. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–1282. - PubMed
-
- Milani M, Ciriello F, Baroni S, et al. FAD-binding site and NADP reactivity in human renalase: a new enzyme involved in blood pressure regulation. J Mol Biol. 2011;411:463–473. - PubMed
-
- Hennebry SC, Eikelis N, Socratous F, et al. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry. 2010;15:234–236. - PubMed
-
- Wu Y, Xu J, Velazquez H, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79:853–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

