Press releases issued by supplements industry organisations and non-industry organisations in response to publication of clinical research findings: a case-control study
- PMID: 24992571
- PMCID: PMC4081644
- DOI: 10.1371/journal.pone.0101533
Press releases issued by supplements industry organisations and non-industry organisations in response to publication of clinical research findings: a case-control study
Abstract
Background: Dietary supplement use is increasing despite lack of evidence of benefits, or evidence of harm. Press releases issued by the supplements industry might contribute to this situation by using 'spin' (strategies to hype or denigrate findings) to distort the results of clinical studies. We assessed press releases issued in response to publication of clinical studies on dietary supplements.
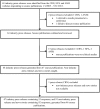
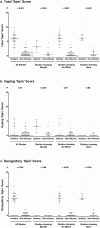
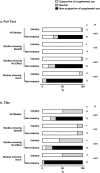
Methods and findings: We analyzed 47 supplements industry press releases and 91 non-industry press releases and news stories, generated in response to 46 clinical studies of dietary supplements published between 1/1/2005 and 5/31/2013. The primary outcome was 'spin' content and direction. We also assessed disposition towards use of dietary supplements, reporting of study information, and dissemination of industry press releases. More supplements industry press releases (100%) contained 'spin' than non-industry media documents (55%, P<0.001). Hyping 'spin' scores were higher in industry than non-industry media documents for studies reporting benefit of supplements (median 'spin' score 3.3, 95% CI 1.0-5.5 vs 0.5, 0-1.0; P<0.001). Denigratory 'spin' scores were higher in industry than non-industry media documents for studies reporting no effect (6.0, 5.0-7.0 vs 0, 0-0; P<0.001) or harm (6.0, 5.5-7.5 vs 0, 0-0.5; P<0.001) from a supplement. Industry press releases advocated supplement use in response to >90% of studies that reported no benefit, or harm, of the supplement. Industry press releases less frequently reported study outcomes, sample size, and estimates of effect size than non-industry media documents (all P<0.001), particularly for studies that reported no benefit of supplements. Industry press releases were referenced by 148 news stories on the websites of 6 organizations that inform manufacturers, retailers and consumers of supplements.
Conclusions: Dietary supplements industry press releases issued in response to clinical research findings are characterized by 'spin' that hypes results that are favourable to supplement use and denigrates results that are not.
Conflict of interest statement
Figures
Similar articles
-
Quality of pharmaceutical industry press releases based on original research.PLoS One. 2008 Jul 30;3(7):e2828. doi: 10.1371/journal.pone.0002828. PLoS One. 2008. PMID: 18716675 Free PMC article.
-
Influence of medical journal press releases on the quality of associated newspaper coverage: retrospective cohort study.BMJ. 2012 Jan 27;344:d8164. doi: 10.1136/bmj.d8164. BMJ. 2012. PMID: 22286507 Free PMC article.
-
Exaggerations and Caveats in Press Releases and Health-Related Science News.PLoS One. 2016 Dec 15;11(12):e0168217. doi: 10.1371/journal.pone.0168217. eCollection 2016. PLoS One. 2016. PMID: 27978540 Free PMC article.
-
Press Releases of Drug-Related Randomized Trial Results Prior to Publication in High-Impact Journals: an Observational Study.J Gen Intern Med. 2023 Nov;38(14):3107-3114. doi: 10.1007/s11606-023-08313-1. Epub 2023 Aug 2. J Gen Intern Med. 2023. PMID: 37532876 Free PMC article.
-
Delays in the Publication of Important Clinical Trial Findings in Oncology.JAMA Oncol. 2018 Jul 12;4(7):e180264. doi: 10.1001/jamaoncol.2018.0264. Epub 2018 Jul 12. JAMA Oncol. 2018. PMID: 29710325 Free PMC article. Review.
Cited by
-
Online Information on Antioxidants: Information Quality Indicators, Commercial Interests, and Ranking by Google.Front Public Health. 2017 Apr 21;5:90. doi: 10.3389/fpubh.2017.00090. eCollection 2017. Front Public Health. 2017. PMID: 28484695 Free PMC article.
-
Errors in NOF meta-analyses of calcium and vitamin D supplements.Osteoporos Int. 2016 Aug;27(8):2637-9. doi: 10.1007/s00198-015-3466-6. Epub 2016 Mar 18. Osteoporos Int. 2016. PMID: 26992924 No abstract available.
-
Challenges of conducting clinical trials of natural products to combat cancer.Clin Adv Hematol Oncol. 2016 Jun;14(6):447-55. Clin Adv Hematol Oncol. 2016. PMID: 27379814 Free PMC article. Review.
-
Outcome Reporting Bias in Government-Sponsored Policy Evaluations: A Qualitative Content Analysis of 13 Studies.PLoS One. 2016 Sep 30;11(9):e0163702. doi: 10.1371/journal.pone.0163702. eCollection 2016. PLoS One. 2016. PMID: 27690131 Free PMC article.
References
-
- Bailey Rl, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173: 355–361. - PubMed
-
- Bacon CJ, Bolland MJ, Ames RW, Siu ATY, Mason BH, et al. (2011) Prevalent dietary supplement use in older New Zealand men. NZ Med J 124: 55–62. - PubMed
-
- Denison HJ, Jameson KA, Syddall HE, Dennison EM, Cooper C, et al. (2012) Patterns of dietary supplement use among older men and women in the UK: findings from the Hertfordshire Cohort Study. J Nutr Health Aging 16: 307–311. - PubMed
-
- Blendon RJ, Benson JM, Botta MD, Weldon KJ (2013) Users’ views of dietary supplements. Arch Intern Med 173: 74–75. - PubMed
-
- Albertazzi P, Steel SA, Clifford E, Bottazzi M (2002) Attitudes towards and use of dietary supplementation in a sample of postmenopausal women. Climacteric 5: 374–382. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

