Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice
- PMID: 24992718
- PMCID: PMC4148050
- DOI: 10.1016/j.vaccine.2014.06.002
Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice
Abstract
An efficacious vaccine is needed to control Chlamydia trachomatis infection. In the murine model of Chlamydia muridarum genital infection, multifunctional mucosal CD4 T cells are the foundation for protective immunity, with antibody playing a secondary role. We previously identified four Chlamydia outer membrane proteins (PmpE, PmpF, PmpG and PmpH) as CD4 T cell vaccine candidates using a dendritic cell-based immunoproteomic approach. We also demonstrated that these four polymorphic membrane proteins (Pmps) individually conferred protection as measured by accelerated clearance of Chlamydia infection in the C57BL/6 murine genital tract model. The major outer membrane protein, MOMP is also a well-studied protective vaccine antigen in this system. In the current study, we tested immunogenicity and protection of a multisubunit recombinant protein vaccine consisting of the four Pmps (PmpEFGH) with or without the major outer membrane protein (MOMP) formulated with a Th1 polarizing adjuvant in C57BL/6, Balb/c and C3H mice. We found that C57BL/6 mice vaccinated with PmpEFGH+MOMP elicited more robust cellular immune responses than mice immunized with individual protein antigens. Pmps elicited more variable cellular immune responses than MOMP among the three strains of mice. The combination vaccine accelerated clearance in the three strains of mice although at different rates. We conclude that the recombinant outer membrane protein combination constitutes a promising first generation Chlamydia vaccine construct that should provide broad immunogenicity in an outbred population.
Keywords: Chlamydia; Immunoproteomics; Recombinant protein; T cell antigens; Vaccine.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


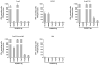
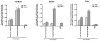
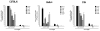

References
-
- Starnbach MN, Roan NR. Conquering sexually transmitted diseases. Nat Rev Immunol. 2008;8:313–7. - PubMed
-
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. - PubMed
-
- Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–7. - PubMed
-
- Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967;63(Suppl):1615–30. - PubMed
-
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

