Adult vascular smooth muscle cells in culture express neural stem cell markers typical of resident multipotent vascular stem cells
- PMID: 24992927
- PMCID: PMC7197789
- DOI: 10.1007/s00441-014-1937-2
Adult vascular smooth muscle cells in culture express neural stem cell markers typical of resident multipotent vascular stem cells
Abstract
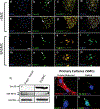
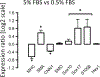
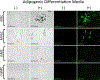
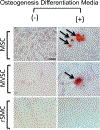
Differentiation of resident multipotent vascular stem cells (MVSCs) or de-differentiation of vascular smooth muscle cells (vSMCs) might be responsible for the SMC phenotype that plays a major role in vascular diseases such as arteriosclerosis and restenosis. We examined vSMCs from three different species (rat, murine and bovine) to establish whether they exhibit neural stem cell characteristics typical of MVSCs. We determined their SMC differentiation, neural stem cell marker expression and multipotency following induction in vitro by using immunocytochemistry, confocal microscopy, fluorescence-activated cell sorting analysis and quantitative real-time polymerase chain reaction. MVSCs isolated from rat aortic explants, enzymatically dispersed rat SMCs and rat bone-marrow-derived mesenchymal stem cells served as controls. Murine carotid artery lysates and primary rat aortic vSMCs were both myosin-heavy-chain-positive but weakly expressed the neural crest stem cell marker, Sox10. Each vSMC line examined expressed SMC differentiation markers (smooth muscle α-actin, myosin heavy chain and calponin), neural crest stem cell markers (Sox10(+), Sox17(+)) and a glia marker (S100β(+)). Serum deprivation significantly increased calponin and myosin heavy chain expression and decreased stem cell marker expression, when compared with serum-rich conditions. vSMCs did not differentiate to adipocytes or osteoblasts following adipogenic or osteogenic inductive stimulation, respectively, or respond to transforming growth factor-β1 or Notch following γ-secretase inhibition. Thus, vascular SMCs in culture express neural stem cell markers typical of MVSCs, concomitant with SMC differentiation markers, but do not retain their multipotency. The ultimate origin of these cells might have important implications for their use in investigations of vascular proliferative disease in vitro.
Figures





Similar articles
-
Embryonic rat vascular smooth muscle cells revisited - a model for neonatal, neointimal SMC or differentiated vascular stem cells?Vasc Cell. 2014 Mar 15;6(1):6. doi: 10.1186/2045-824X-6-6. Vasc Cell. 2014. PMID: 24628920 Free PMC article.
-
Inhibitory role of reactive oxygen species in the differentiation of multipotent vascular stem cells into vascular smooth muscle cells in rats: a novel aspect of traditional culture of rat aortic smooth muscle cells.Cell Tissue Res. 2015 Oct;362(1):97-113. doi: 10.1007/s00441-015-2193-9. Epub 2015 May 29. Cell Tissue Res. 2015. PMID: 26022334
-
Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells.Cells. 2025 Apr 17;14(8):611. doi: 10.3390/cells14080611. Cells. 2025. PMID: 40277936 Free PMC article.
-
Circulating smooth muscle progenitor cells in arterial remodeling.J Mol Cell Cardiol. 2011 Feb;50(2):273-9. doi: 10.1016/j.yjmcc.2010.10.030. Epub 2010 Nov 1. J Mol Cell Cardiol. 2011. PMID: 21047514 Review.
-
Origin and differentiation of vascular smooth muscle cells.J Physiol. 2015 Jul 15;593(14):3013-30. doi: 10.1113/JP270033. Epub 2015 Jun 9. J Physiol. 2015. PMID: 25952975 Free PMC article. Review.
Cited by
-
Sox10+ Cells Contribute to Vascular Development in Multiple Organs-Brief Report.Arterioscler Thromb Vasc Biol. 2017 Sep;37(9):1727-1731. doi: 10.1161/ATVBAHA.117.309774. Epub 2017 Jul 27. Arterioscler Thromb Vasc Biol. 2017. PMID: 28751573 Free PMC article.
-
Hedgehog and Resident Vascular Stem Cell Fate.Stem Cells Int. 2015;2015:468428. doi: 10.1155/2015/468428. Epub 2015 May 6. Stem Cells Int. 2015. PMID: 26064136 Free PMC article. Review.
-
Nox, Reactive Oxygen Species and Regulation of Vascular Cell Fate.Antioxidants (Basel). 2017 Nov 14;6(4):90. doi: 10.3390/antiox6040090. Antioxidants (Basel). 2017. PMID: 29135921 Free PMC article. Review.
-
Disease-Relevant Single Cell Photonic Signatures Identify S100β Stem Cells and their Myogenic Progeny in Vascular Lesions.Stem Cell Rev Rep. 2021 Oct;17(5):1713-1740. doi: 10.1007/s12015-021-10125-x. Epub 2021 Mar 17. Stem Cell Rev Rep. 2021. PMID: 33730327 Free PMC article.
-
Secreted miR-27a Induced by Cyclic Stretch Modulates the Proliferation of Endothelial Cells in Hypertension via GRK6.Sci Rep. 2017 Jan 20;7:41058. doi: 10.1038/srep41058. Sci Rep. 2017. PMID: 28106155 Free PMC article.
References
-
- Babij P, Kawamoto S, White S, Adelstein RS, Periasamy M (1992) Differential expression of SM1 and SM2 myosin isoforms in cultured vascular smooth muscle. Am J Physiol 262:C607–C613 - PubMed
-
- Bukovsky A (2009) Sex steroid-mediated reprogramming of vascular smooth muscle cells to stem cells and neurons: possible utilization of sex steroid combinations for regenerative treatment without utilization of in vitro developed stem cells. Cell Cycle 8:4079–4084 - PubMed
-
- Chamley JH, Campbell GR, Burnstock G (1974) Dedifferentiation, redifferentiation and bundle formation of smooth muscle cells in tissue culture: the influence of cell number and nerve fibres. J Embryol Exp Morphol 32:297–323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

