Plasma fluoride level as a predictor of voriconazole-induced periostitis in patients with skeletal pain
- PMID: 24992954
- PMCID: PMC4351342
- DOI: 10.1093/cid/ciu513
Plasma fluoride level as a predictor of voriconazole-induced periostitis in patients with skeletal pain
Abstract
Background: Voriconazole is a triazole antifungal medication used for prophylaxis or to treat invasive fungal infections. Inflammation of the periosteum resulting in skeletal pain, known as periostitis, is a reported side effect of long-term voriconazole therapy. The trifluorinated molecular structure of voriconazole suggests a possible link between excess fluoride and periostitis, as elevated blood fluoride has been reported among patients with periostitis who received voriconazole.
Methods: Two hundred sixty-four patients from Michigan were impacted by the multistate outbreak of fungal infections as a result of contaminated methylprednisolone injections. A retrospective study was conducted among 195 patients who received voriconazole therapy at St Joseph Mercy Hospital during this outbreak. Twenty-eight patients who received both bone scan and plasma fluoride measurements for skeletal pain were included in the statistical analyses. Increased tracer uptake on bone scan was considered positive for periostitis. The primary outcome measure was the correlation between plasma fluoride and bone scan results.
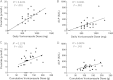
Results: Blood fluoride (P < .001), alkaline phosphatase (P = .020), daily voriconazole dose (P < .001), and cumulative voriconazole dose (P = .027) were significantly elevated in patients who had periostitis compared with those who did not. Discontinuation or dose reduction of voriconazole resulted in improvement of pain in 89% of patients.
Conclusions: High plasma fluoride levels coupled with skeletal pain among patients who are on long-term voriconazole therapy is highly suggestive of periostitis. Initial measurement of fluoride may be considered when bone scan is not readily available. Early detection should be sought, as discontinuation of voriconazole is effective at reversing the disease.
Keywords: contaminated methylprednisolone injection; fluorosis; fungal infection; periostitis; voriconazole.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Smith RM, Schaefer MK, Kainer MA, et al. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med. 2013;369:1598–609. - PubMed
-
- Chiller TM, Roy M, Nguyen D, et al. Clinical findings for fungal infections caused by methylprednisolone injections. N Engl J Med. 2013;369:1610–9. - PubMed
-
- Malani AN, Vandenberg DM, Singal B, et al. Magnetic resonance imaging screening to identify spinal and paraspinal infections associated with injections of contaminated methylprednisolone acetate. JAMA. 2013;309:2465–72. - PubMed
-
- Centers for Disease Control and Prevention. Multistate fungal meningitis outbreak investigation. Available at: http://www.cdc.gov/hai/outbreaks/meningitis.html . Accessed 12 July 2014.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

