Modulation of chemokine gradients by apheresis redirects leukocyte trafficking to different compartments during sepsis, studies in a rat model
- PMID: 24992991
- PMCID: PMC4227131
- DOI: 10.1186/cc13969
Modulation of chemokine gradients by apheresis redirects leukocyte trafficking to different compartments during sepsis, studies in a rat model
Abstract
Introduction: Prior work suggests that leukocyte trafficking is determined by local chemokine gradients between the nidus of infection and the plasma. We recently demonstrated that therapeutic apheresis can alter immune mediator concentrations in the plasma, protect against organ injury, and improve survival. Here we aimed to determine whether the removal of chemokines from the plasma by apheresis in experimental peritonitis changes chemokine gradients and subsequently enhances leukocyte localization into the infected compartment, and away from healthy tissues.
Methods: In total, 76 male adult Sprague-Dawley rats weighing 400 g to 600 g were included in this study. Eighteen hours after inducing sepsis by cecal ligation and puncture, we randomized these rats to apheresis or sham treatment for 4 hours. Cytokines, chemokines, and leukocyte counts from blood, peritoneal cavity, and lung were measured. In a separate experiment, we labeled neutrophils from septic donor animals and injected them into either apheresis or sham-treated animals. All numeric data with normal distributions were compared with one-way analysis of variance, and numeric data not normally distributed were compared with the Mann-Whitney U test.
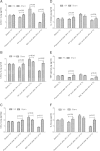
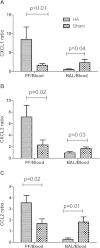
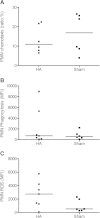
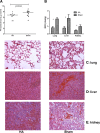
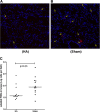
Results: Apheresis significantly removed plasma cytokines and chemokines, increased peritoneal fluid-to-blood chemokine (C-X-C motif ligand 1, ligand 2, and C-C motif ligand 2) ratios, and decreased bronchoalveolar lavage fluid-to-blood chemokine ratios, resulting in enhanced leukocyte recruitment into the peritoneal cavity and improved bacterial clearance, but decreased recruitment into the lung. Apheresis also reduced myeloperoxidase activity and histologic injury in the lung, liver, and kidney. These Labeled donor neutrophils exhibited decreased localization in the lung when infused into apheresis-treated animals.
Conclusions: Our results support the concept of chemokine gradient control of leukocyte trafficking and demonstrate the efficacy of apheresis to target this mechanism and reduce leukocyte infiltration into the lung.
Figures

References
-
- Alves-Filho JC, Sônego F, Soto FO, Freitas A, Verri WA, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. - PubMed
-
- Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. - PubMed
-
- Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. - PubMed
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
-
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

