Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy
- PMID: 24993324
- PMCID: PMC4289822
- DOI: 10.1038/eye.2014.144
Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy
Abstract
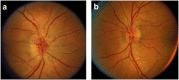
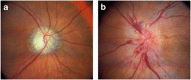
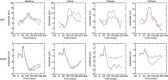
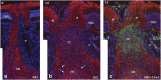
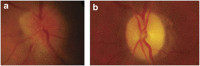
Nonarteritic anterior ischaemic optic neuropathy (NAION) is the most common acute optic neuropathy in patients over the age of 50 and is the second most common cause of permanent optic nerve-related visual loss in adults after glaucoma. Patients typically present with acute, painless, unilateral loss of vision associated with a variable visual field defect, a relative afferent pupillary defect, a swollen, hyperaemic optic disc, and one or more flame-shaped peripapillary retinal haemorrhages. The pathogenesis of this condition is unknown, but it occurs primarily in patients with structurally small optic discs that have little or no cup and a variety of underlying vascular disorders that may or may not be known at the time of visual loss. There is no consistently beneficial medical or surgical treatment for the condition, but there are now animal models that allow testing of various potential therapies. About 40% of patients experience spontaneous improvement in visual acuity. Patients in whom NAION occurs in one eye have a 15-19% risk of developing a similar event in the opposite eye over the subsequent 5 years.
Figures



References
-
- Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:103–107. - PubMed
-
- Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy: population-based study in the State of Missouri and Los Angeles County, California. J Neuroophthalmol. 1994;14:38–44. - PubMed
-
- Guyer DR, Miller NR, Auer CL, Fine SL. The risk of cerebrovascular and cardiovascular disease in patients with anterior ischemic optic neuropathy. Arch Ophthalmol. 1985;103:1136–1142. - PubMed
-
- Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96:478–483. - PubMed
-
- Ischemic Optic Neuropathy Decompression Trial Research Group Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol. 1996;114:1366–1374. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

