Biological products for the treatment of psoriasis: therapeutic targets, pharmacodynamics and disease-drug-drug interaction implications
- PMID: 24993574
- PMCID: PMC4147058
- DOI: 10.1208/s12248-014-9637-0
Biological products for the treatment of psoriasis: therapeutic targets, pharmacodynamics and disease-drug-drug interaction implications
Abstract
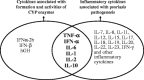
Psoriasis is a chronic inflammatory skin disease condition that involves altered expression of a broad spectrum of proinflammatory cytokines which are associated with activation of T cells and proliferation of keratinocytes. Currently approved biological products for psoriasis treatment fall into two main classes: cytokine modulators and biologics targeting T cells. In psoriatic patients, elevated levels of proinflammatory cytokines are observed. Elevated proinflammatory cytokines can suppress some cytochrome P450 (CYP) enzymes, and the treatment of psoriasis with biological products can reduce proinflammatory cytokine levels. Therefore, the exposure of CYP substrate drugs is anticipated to be affected by the psoriasis disease resulting in a higher exposure than in healthy state (named disease-drug interaction) as well as by the biological treatments due to disease improvements resulting in a decrease in exposure (named disease-drug-drug interaction, disease-DDI). However, the quantitative impact on CYP substrate exposure due to disease or due to treatment with biological products remains to be evaluated. The objective of the current review is to provide an overview of the therapeutic targets and cytokine-related pharmacodynamic effects of biological products in psoriasis treatment with a particular focus on their implications for disease-DDI. The clinical study design considerations for psoriasis disease-DDI evaluation are also discussed.
Figures

References
-
- Sivamani RK, Goodarzi H, Garcia MS, Raychaudhuri SP, Wehrli LN, Ono Y, et al. Biologic Therapies in the Treatment of Psoriasis: A Comprehensive Evidence-Based Basic Science and Clinical Review and a Practical Guide to Tuberculosis Monitoring. Clin Rev Allergy Immunol 2012 Feb 5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

