Substrate-water exchange in photosystem II is arrested before dioxygen formation
- PMID: 24993602
- PMCID: PMC4102119
- DOI: 10.1038/ncomms5305
Substrate-water exchange in photosystem II is arrested before dioxygen formation
Abstract
Light-driven oxidation of water into dioxygen, catalysed by the oxygen-evolving complex (OEC) in photosystem II, is essential for life on Earth and provides the blueprint for devices for producing fuel from sunlight. Although the structure of the OEC is known at atomic level for its dark-stable state, the mechanism by which water is oxidized remains unsettled. Important mechanistic information was gained in the past two decades by mass spectrometric studies of the H2(18)O/H2(16)O substrate-water exchange in the four (semi) stable redox states of the OEC. However, until now such data were not attainable in the transient states formed immediately before the O-O bond formation. Using modified photosystem II complexes displaying up to 40-fold slower O2 production rates, we show here that in the transient S3YZ state the substrate-water exchange is dramatically slowed as compared with the earlier S states. This further constrains the possible sites for substrate-water binding in photosystem II.
Figures


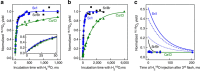
 state at m/z=34. Symbols mark the data points (green triangles, Ca/Cl-PSII; black squares, Sr/Br-PSII; blue circles, Sr/I-PSII), while full lines are fits representing the fast and slow substrate–water exchange (a,b; for rate constants see Table 1) or simulations of the expected experimental outcome assuming the exchange rates are identical in the S3+YZ and
state at m/z=34. Symbols mark the data points (green triangles, Ca/Cl-PSII; black squares, Sr/Br-PSII; blue circles, Sr/I-PSII), while full lines are fits representing the fast and slow substrate–water exchange (a,b; for rate constants see Table 1) or simulations of the expected experimental outcome assuming the exchange rates are identical in the S3+YZ and  states (c). The blue dashed and dotted lines in c represent simulations where either the slow (dashed) or fast (dotted) rate of exchange was set to be 1,000 times slower than that measured in the S3+YZ state (Table 1). All data points (n=1) are normalized to values reached after complete isotopic equilibration. Each time course was measured once, but consists of many separately measured data points that were in part obtained on different days.
states (c). The blue dashed and dotted lines in c represent simulations where either the slow (dashed) or fast (dotted) rate of exchange was set to be 1,000 times slower than that measured in the S3+YZ state (Table 1). All data points (n=1) are normalized to values reached after complete isotopic equilibration. Each time course was measured once, but consists of many separately measured data points that were in part obtained on different days.Similar articles
-
Structure of photosystem II and substrate binding at room temperature.Nature. 2016 Dec 15;540(7633):453-457. doi: 10.1038/nature20161. Epub 2016 Nov 21. Nature. 2016. PMID: 27871088 Free PMC article.
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Structures and energetics for O2 formation in photosystem II.Acc Chem Res. 2009 Dec 21;42(12):1871-80. doi: 10.1021/ar900117k. Acc Chem Res. 2009. PMID: 19856959
-
Biological water oxidation.Acc Chem Res. 2013 Jul 16;46(7):1588-96. doi: 10.1021/ar3003249. Epub 2013 Mar 18. Acc Chem Res. 2013. PMID: 23506074 Review.
-
Substrate water exchange in the S2 state of photosystem II is dependent on the conformation of the Mn4Ca cluster.Phys Chem Chem Phys. 2020 Jun 21;22(23):12894-12908. doi: 10.1039/d0cp01380c. Epub 2020 May 6. Phys Chem Chem Phys. 2020. PMID: 32373850
Cited by
-
From manganese oxidation to water oxidation: assembly and evolution of the water-splitting complex in photosystem II.Photosynth Res. 2022 May;152(2):107-133. doi: 10.1007/s11120-022-00912-z. Epub 2022 Apr 9. Photosynth Res. 2022. PMID: 35397059 Review.
-
Water oxidation in photosystem II.Photosynth Res. 2019 Oct;142(1):105-125. doi: 10.1007/s11120-019-00648-3. Epub 2019 Jun 11. Photosynth Res. 2019. PMID: 31187340 Free PMC article. Review.
-
Assignment of the slowly exchanging substrate water of nature's water-splitting cofactor.Proc Natl Acad Sci U S A. 2024 Mar 12;121(11):e2319374121. doi: 10.1073/pnas.2319374121. Epub 2024 Mar 4. Proc Natl Acad Sci U S A. 2024. PMID: 38437550 Free PMC article.
-
Coemissive luminescent nanoparticles combining aggregation-induced emission and quenching dyes prepared in continuous flow.Nat Commun. 2022 Oct 13;13(1):6034. doi: 10.1038/s41467-022-33857-x. Nat Commun. 2022. PMID: 36229467 Free PMC article.
-
Unravelling Mn4Ca cluster vibrations in the S1, S2 and S3 states of the Kok-Joliot cycle of photosystem II.Phys Chem Chem Phys. 2024 Jul 31;26(30):20598-20609. doi: 10.1039/d4cp01307g. Phys Chem Chem Phys. 2024. PMID: 39037338 Free PMC article.
References
-
- Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J. & Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004). - PubMed
-
- Guskov A. et al. Cyanobacterial photosystem II at 2.9 Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Biol. Mol. Biol. 16, 334–342 (2009). - PubMed
-
- Umena Y., Kawakami K., Shen J. R. & Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–61 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

