Ribonomic analysis of human DZIP1 reveals its involvement in ribonucleoprotein complexes and stress granules
- PMID: 24993635
- PMCID: PMC4091656
- DOI: 10.1186/1471-2199-15-12
Ribonomic analysis of human DZIP1 reveals its involvement in ribonucleoprotein complexes and stress granules
Abstract
Background: DZIP1 (DAZ-interacting protein 1) has been described as a component of the Hh signaling pathway with a putative regulatory role in ciliogenesis. DZIP1 interacts with DAZ RNA binding proteins in embryonic stem cells and human germ cells suggesting a role in mRNA regulation.
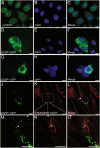
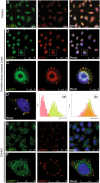
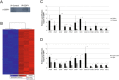
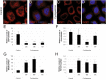
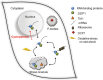
Results: We investigated DZIP1 function in HeLa cells and its involvement in ribonucleoprotein complexes. DZIP1 was predominantly located in granules in the cytoplasm. Under oxidative stress conditions, DZIP1 re-localized to stress granules. DZIP appears to be important for the formation of stress granules during the stress response. We used immunoprecipitation assays with antibodies against DZIP1 and microarray hybridization to identify mRNAs associated with DZIP1. The genetic networks formed by the DZIP1-associated mRNAs were involved in cell cycle and gene expression regulation. DZIP1 is involved in the Hedgehog signaling pathway. We used cyclopamine, a specific inhibitor of this pathway, to analyze the expression of DZIP1 and its associated mRNAs. The abundance of DZIP1-associated mRNAs increased with treatment; however, the silencing or overexpression of DZIP1 in HeLa cells had no effect on the accumulation of the associated mRNAs. Polysomal profile analysis by sucrose gradient centrifugation demonstrated the presence of DZIP1 in the polysomal fraction.
Conclusions: Our results suggest that DZIP1 is part of an RNP complex that occupies various subcellular locations. The diversity of the mRNAs associated with DZIP1 suggests that this protein is a component of different RNPs associated with translating polysomes and with RNA granules.
Figures

Similar articles
-
Stabilization of speckle-type POZ protein (Spop) by Daz interacting protein 1 (Dzip1) is essential for Gli turnover and the proper output of Hedgehog signaling.J Biol Chem. 2013 Nov 8;288(45):32809-32820. doi: 10.1074/jbc.M113.512962. Epub 2013 Sep 26. J Biol Chem. 2013. PMID: 24072710 Free PMC article.
-
Centrosomal protein DZIP1 regulates Hedgehog signaling by promoting cytoplasmic retention of transcription factor GLI3 and affecting ciliogenesis.J Biol Chem. 2013 Oct 11;288(41):29518-29. doi: 10.1074/jbc.M113.492066. Epub 2013 Aug 16. J Biol Chem. 2013. PMID: 23955340 Free PMC article.
-
Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA.J Cell Sci. 2010 Feb 1;123(Pt 3):369-83. doi: 10.1242/jcs.055897. Epub 2010 Jan 5. J Cell Sci. 2010. PMID: 20053637
-
Relationship of GW/P-bodies with stress granules.Adv Exp Med Biol. 2013;768:197-211. doi: 10.1007/978-1-4614-5107-5_12. Adv Exp Med Biol. 2013. PMID: 23224972 Free PMC article. Review.
-
mRNP granules. Assembly, function, and connections with disease.RNA Biol. 2014;11(8):1019-30. doi: 10.4161/15476286.2014.972208. RNA Biol. 2014. PMID: 25531407 Free PMC article. Review.
Cited by
-
Crosstalk between Hedgehog pathway and energy pathways in human adipose-derived stem cells: A deep sequencing analysis of polysome-associated RNA.Sci Rep. 2018 May 30;8(1):8411. doi: 10.1038/s41598-018-26533-y. Sci Rep. 2018. PMID: 29849100 Free PMC article.
-
DZIP1 Expression as a Prognostic Marker in Gastric Cancer: A Bioinformatics-Based Analysis.Pharmgenomics Pers Med. 2021 Sep 16;14:1151-1168. doi: 10.2147/PGPM.S325701. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 34557018 Free PMC article.
-
Response to stress in biological disorders: Implications of stress granule assembly and function.Cell Prolif. 2021 Aug;54(8):e13086. doi: 10.1111/cpr.13086. Epub 2021 Jun 25. Cell Prolif. 2021. PMID: 34170048 Free PMC article. Review.
-
DAZ Family Proteins, Key Players for Germ Cell Development.Int J Biol Sci. 2015 Aug 15;11(10):1226-35. doi: 10.7150/ijbs.11536. eCollection 2015. Int J Biol Sci. 2015. PMID: 26327816 Free PMC article. Review.
-
RNA-Binding RING E3-Ligase DZIP3/hRUL138 Stabilizes Cyclin D1 to Drive Cell-Cycle and Cancer Progression.Cancer Res. 2021 Jan 15;81(2):315-331. doi: 10.1158/0008-5472.CAN-20-1871. Epub 2020 Oct 16. Cancer Res. 2021. PMID: 33067265 Free PMC article.
References
-
- Moore F, Jaruzelska J, Dorfman D, Reijo-Pera R. Identification of a novel gene, DZIP (DAZ-interacting protein), that encodes a protein that interacts with DAZ (deleted in azoospermia) and is expressed in embryonic stem cells and germ cells. Genomics. 2004;83(5):834–843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

