The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495 and Ser1177
- PMID: 24993645
- PMCID: PMC4122979
- DOI: 10.1042/BSR20140079
The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495 and Ser1177
Abstract
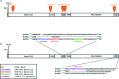
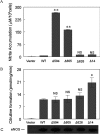
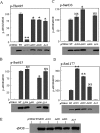
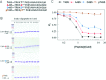
NO production catalysed by eNOS (endothelial nitric-oxide synthase) plays an important role in the cardiovascular system. A variety of agonists activate eNOS through the Ser1177 phosphorylation concomitant with Thr495 dephosphorylation, resulting in increased ·NO production with a basal level of calcium. To date, the underlying mechanism remains unclear. We have previously demonstrated that perturbation of the AIE (autoinhibitory element) in the FMN-binding subdomain can also lead to eNOS activation with a basal level of calcium, implying that the AIE might regulate eNOS activation through modulating phosphorylation at Thr495 and Ser1177. Here we generated stable clones in HEK-293 (human embryonic kidney 293) cells with a series of deletion mutants in both the AIE (Δ594-604, Δ605-612 and Δ626-634) and the C-terminal tail (Δ14; deletion of 1164-1177). The expression of Δ594-604 and Δ605-612 mutants in non-stimulated HEK-293 cells substantially increased nitrate/nitrite release into the culture medium; the other two mutants, Δ626-634 and Δ1164-1177, displayed no significant difference when compared with WTeNOS (wild-type eNOS). Intriguingly, mutant Δ594-604 showed close correlation between Ser1177 phosphorylation and Thr495 dephosphorylation, and NO production. Our results have indicated that N-terminal portion of AIE (residues 594-604) regulates eNOS activity through coordinated phosphorylation on Ser1177 and Thr495.
Figures



References
-
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. - DOI - PMC - PubMed
-
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11141–11145. doi: 10.1073/pnas.89.23.11141. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

